Nov 11 2019
NUS scientists have demonstrated a strain-induced structural rearrangement of one-dimensional (1D) metal-organic molecular chains for potential use in fabricating functional nanostructures.
The synthesis of functional materials at the molecular level can potentially be used to develop nanostructures for applications requiring specially tailored electronic and magnetic properties. This is usually achieved by using thermal- or photo-triggered chemical transformations. The use of mechanical strains to trigger chemical transformations provides a new way to fabricate nanostructures with unique properties.
A research team led by Prof LU Jiong from the Department of Chemistry, NUS has demonstrated that the strain created between one-dimensional (1D) metal-organic chains (MOCs) and their underlying substrate can trigger isomeric transformations which can result in a new molecular structure. In isomeric transformations, the atoms in the molecule rearrange themselves, producing a structurally different molecule but having the same atoms. By creating appropriate strain conditions in the material, the resulting isomeric transformations can potentially be used to fabricate functional nanostructures.
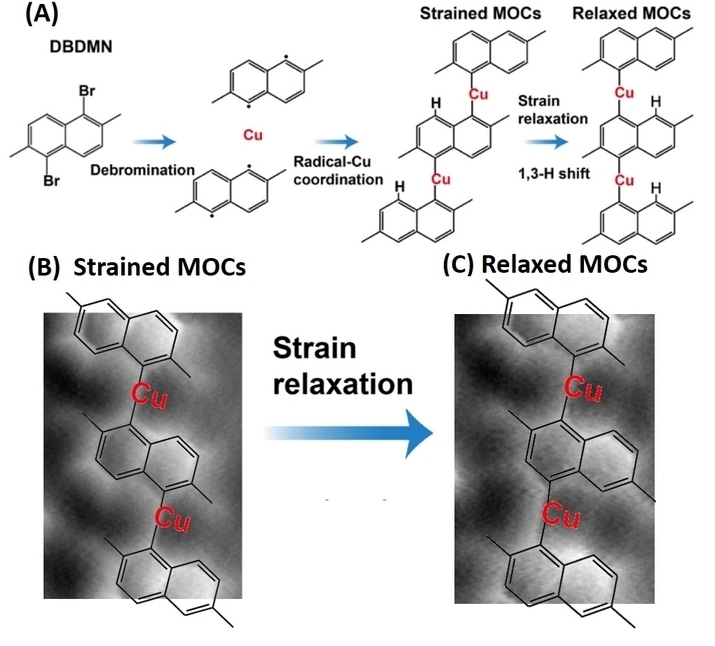
A rationally designed compound, known as 1,5-dibromo-2,6-dimethylnaphthalene (DBDMN) was synthesised by Prof WU Jishan’s group from Department of Chemistry, NUS. Prof Lu’s team deposited this compound onto a catalytically active copper surface to form 1D MOCs (Figure A). When subjected to heat treatment and cooling at room conditions to reduce the stress in the material, the team found that the MOCs underwent skeletal isomeric rearrangements. The structure of the MOCs at submolecular resolution prior and after the transformation was captured using non-contact atomic force microscopy (Figures B and C). The images show that during the transformation process, the C-H bonds become chemically active with rearrangements of coordination bonds.
The team’s experimental results together with theoretical calculations carried out by Prof Pavel Jelínek’s group from the Institute of Physics, Czech Academy of Science, Czech Republic show that the reduction in the substrate-induced internal strain on the MOCs is the key factor which is causing the molecular rearrangement in the material.
Prof Lu said, "We envisage that our findings on the strain-induced structural rearrangement in one-dimensional material systems will enrich the available toolbox for on-surface synthesis of novel functional materials and quantum nanostructures.”
Figure (A) provides a schematic illustration of the synthesis of metal-organic chains (MOCs) and their structural relaxation on a copper substrate. The compound 1,5-dibromo-2,6-dimethylnaphthalene (DBDMN) which is deposited undergoes a debromination process to form one-dimensional (1D) metal-organic chains (MOCs). Annealing at room temperature causes a rearrangement of the molecular structure. Figures (B) and (C) are the images obtained by non-contact atomic force microscopy showing the molecular structure under strained and relaxed conditions. [Credit: ANGEWANDTE CHEMIE]
Reference
Telychko M; Su J; Gallardo A; Gu Y; Mendieta-Moreno JI; Qi D; Tadich A; Song S; Lyu P; Qiu Z; Fang H; Koh MJ; Wu J*; Jelínek P*; Lu J* “Strain-induced isomerization in one-dimensional metal-organic chains” ANGEWANDTE CHEMIE DOI: 10.1002/anie.201909074 Published: 2019.