Image Credit: Tokyo Institute of Technology.
With this technique, a special crystalline oxide comprising phosphorus, sulfur, and zirconium can be produced. This crystalline oxide has two separate mechanisms of negative thermal expansion. This material is also the first to exhibit this property, and its application may help prevent damage to composite materials, like computer chip components, that experience sudden temperature changes.
Upon heating, most of the materials tend to expand as the atoms get separated. The coefficient of thermal expansion (CTE) is used to measure the expansibility of materials under heat. A majority of the existing industry-grade materials have a positive CTE, which negatively impacts their performance when subjected to more 'extreme' temperatures.
But certain materials tend to face the opposite effect, reducing in size at higher temperatures. This strange process, called negative thermal expansion, may help solve the issue of heat damage caused to composite materials.
A research team from the Tokyo Institute of Technology under the guidance of Associate Professor Toshihiro Isobe has been investigating materials that have negative CTE.
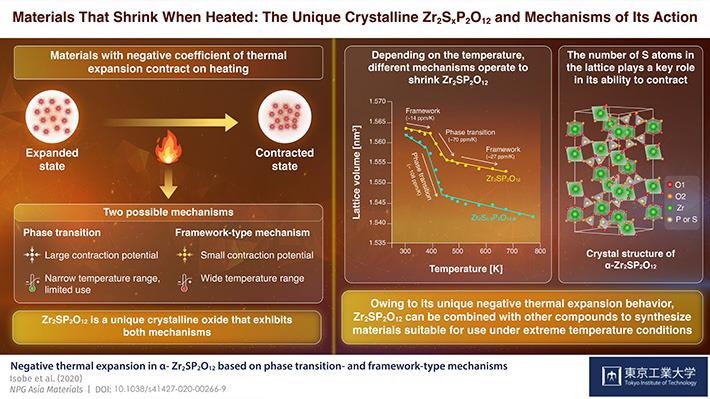
"Negative thermal expansion behavior can be primarily attributed to two types of mechanisms, phase transition and framework-type mechanism."
Toshihiro Isobe, Associate Professor, Tokyo Institute of Technology
The two mechanisms are used in industrial applications as both have advantages and disadvantages. While phase transition-type materials contain large negative CTEs, they also have narrow usable temperature ranges, which restrict their operational use, especially as fillers in composite materials.
On the other hand, framework-type materials exhibit thermal shrinkage across a broad temperature range, but since they have small absolute CTE values, they are needed in huge quantities to obtain the preferred outcome.
For many years, investigators have been looking for an appropriate compromise between the two, but materials that can experience both mechanisms of negative thermal expansion have never been reported so far.
In their latest study, published in the NPG Asia Materials journal, Dr Isobe and his team have reported a technique to produce a new crystalline oxide composed of phosphorus, sulfur, and zirconium, explaining its characteristics. This crystal, whose chemical formula is Zr2SP2O12, is outlined by Dr Isobe as 'a negative CTE material which displays both transition- and framework-type mechanisms when heated.'
The researchers discovered that, while Zr2SP2O12 displays both mechanisms of negative thermal mechanism stated before, one mechanism might be dominant at a specified temperature. For example, between 393 °K (roughly 120 °C) and 453°K (approximately 180 °C), the material shrunk quickly and certain structural units were also deformed, which denotes a phase transition.
However, above and below this range of temperature, the contraction was not as significant, and the team instead noted slight variations in the angle and length of bonds present between atoms, a property of framework-type mechanism.
The team also observed a fascinating phenomenon. They noted that the crystals comprising fewer sulfur atoms in the lattice were deformed more easily at the time of phase transition (120 °C to 180 °C), leading to a larger contraction of the material (higher negative CTE). This can help produce Zr2SP2O12 crystals that have the preferred CTE for particular applications.
This latest crystalline material and its production mechanism could lay the basis for producing compounds with an analogous dual mechanism. This would allow material engineers to choose compounds with particular properties to customize the performance of produced materials to particular operational conditions.
Atomic rearrangement that looks shrinking when warmed
Atomic rearrangement that looks shrinking when warmed. The atomic waltz in the crystalline lattice in this interactive video helps understand this fascinating mechanism of atomic rearrangement in response to heat more clearly. Video Credit: Toshihiro Isobe.
Journal Reference:
Isobe, T., et al. (2020) Negative thermal expansion in α-Zr2SP2O12 based on phase transition- and framework-type mechanisms. NPG Asia Materials. doi.org/10.1038/s41427-020-00266-9