Jun 11 2021
An exploratory analysis of the behavior of materials with preferred electric characteristics led to the discovery of a structural phase of 2D materials.
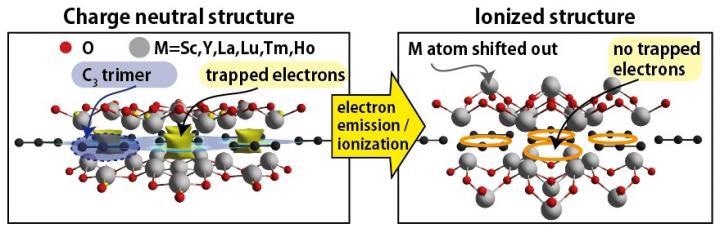 Yellow isosurfaces on the left panel indicate electrons localized in-between the C3 trimers. The ionized structure on the right has no trapped electrons, and some of the M atoms have been largely displaced. This displacement of the M atoms again significantly stabilizes the ionized structure. Image Credit: Soungmin Bae and Hannes Raebiger.
Yellow isosurfaces on the left panel indicate electrons localized in-between the C3 trimers. The ionized structure on the right has no trapped electrons, and some of the M atoms have been largely displaced. This displacement of the M atoms again significantly stabilizes the ionized structure. Image Credit: Soungmin Bae and Hannes Raebiger.
Electrides are a new class of materials in which electrons take up a space that is normally reserved for ions or atoms instead of orbiting the nucleus of an ion or atom. The tunable, stable, low-energy materials can have potential applications in nanotechnologies.
The international team of researchers, headed by Hannes Raebiger, associate professor in the Department of Physics at Yokohama National University in Japan, have reported their findings as a frontispiece in the Advanced Functional Materials journal on June 10th, 2021.
The researchers initially set out to gain a better understanding of the basic features of a 2D system called Sc2CO2. The system, which contains two atoms of metallic scandium, two atoms of oxygen and one atom of carbon, belongs to a group of chemical compounds collectively known as MXenes. The system is typically made up of a one-atom-thick nitrogen or carbon layer closely packed between metal layers and dotted with fluorine or oxygen atoms.
MXene Sc2CO2 particularly caught the researchers’ interest, because it was predicted that when the system is organized into a hexagonal phase, it would have desirable electrical characteristics.
Despite these fascinating predictions of hexagonal phases of Sc2CO2, we are not aware of its successful fabrication as of yet. Analyzing its fundamental properties, we discovered a completely new structural phase.
Soungmin Bae, Study First Author and Researcher, Department of Physics, Yokohama National University
New electride materials resulted from the new structural phase. The atomic-thin 2D structural phase has been described as tiled shapes creating the core carbon plane. The formerly projected form was a hexagon, with one carbon atom in the center and one at each vertex. The novel materials have a shape similar to a rhombus, with electrons located at the vertices and a carbon trimer — three carbon atoms in a row — in the center.
Carbon is one of the most common materials on our planet, and quite important for living beings, but it is hardly ever found as trimers. The closest place where carbon trimers are typically found is interstellar space.
Hannes Raebiger, Associate Professor, Department of Physics, Yokohama National University
While the overall shape is less symmetric than the earlier reported hexagonal arrangement, it is more symmetric with respect to the central plane. According to Raebiger, this structure has distinct properties because of the advent of the new class of electrides.
Electrides contain electrons as a structural unit and often are extremely good electrical conductors. The present family of electrides are insulators, and while most insulators can be made conductive by adding or removing electrons, these materials simply become more insulating.
Hannes Raebiger, Associate Professor, Department of Physics, Yokohama National University
MXenes are particularly appealing as a material because they can be reconfigured with other kinds of metallic elements to provide a plethora of properties, such as adjustable conductivity, various types of magnetism, and/or function as catalysts to speed chemical reactions.
MXenes are also ultra-thin sheets that have a thickness of only a few atoms, that is, 2D materials. The recently identified electrides contain electrons in lattice spaces between ions and atoms that can be instantly released into the surrounding space, for example, the electron sources for huge particle accelerators. They can also be borrowed to accelerate a specific chemical reaction.
“We made this discovery because we wanted to understand how these materials work better. If you encounter something you don't understand, dig deeper,” concluded Bae.
Co-authors of the study include William Espinosa-García and Gustavo M. Dalpian, Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Brazil; Yoon-Gu Kang and Myung Joon Han, Department of Physics, Korea Advanced Institute of Science and Technology; Juho Lee and Yong-Hoon Kim, Department of Electrical Engineering, Korea Advanced Institute of Science and Technology; Noriyuki Egawa, Kazuaki Kuwahata, and Kaoru Ohno, Department of Physics at Yokohama National University.
Other co-authors included Mohammad Khazaei and Hideo Hosono, Materials Research Center for Element Strategy, Tokyo Institute of Technology. Espinosa-García is also affiliated with Grupo de investigación en Modelamienot y Simulación Computacional, Facultad de Ingenierías, Universidad de San Buenaventura-Medellín.
The study was funded by the Iwaki Scholarship Foundation; São Paulo Research Foundation; Korea's National Research Foundation, Ministry of Science and ICT and Ministry of Education; KAIST (formerly the Korea Advanced Institute of Science and Technology); and Samsung Research Funding & Incubation Center of Samsung Electronics.
Journal Reference:
Bae, S., et al. (2021) MXene Phase with C3 Structure Unit: A Family of 2D Electrides. Advanced Functional Materials. doi.org/10.1002/adfm.202100009.