Researchers generally select a research problem carefully before embarking on their research. Then, they devise a suitable plan to solve it and finally execute that plan. However, occasionally, accidental discoveries can take place during the research.
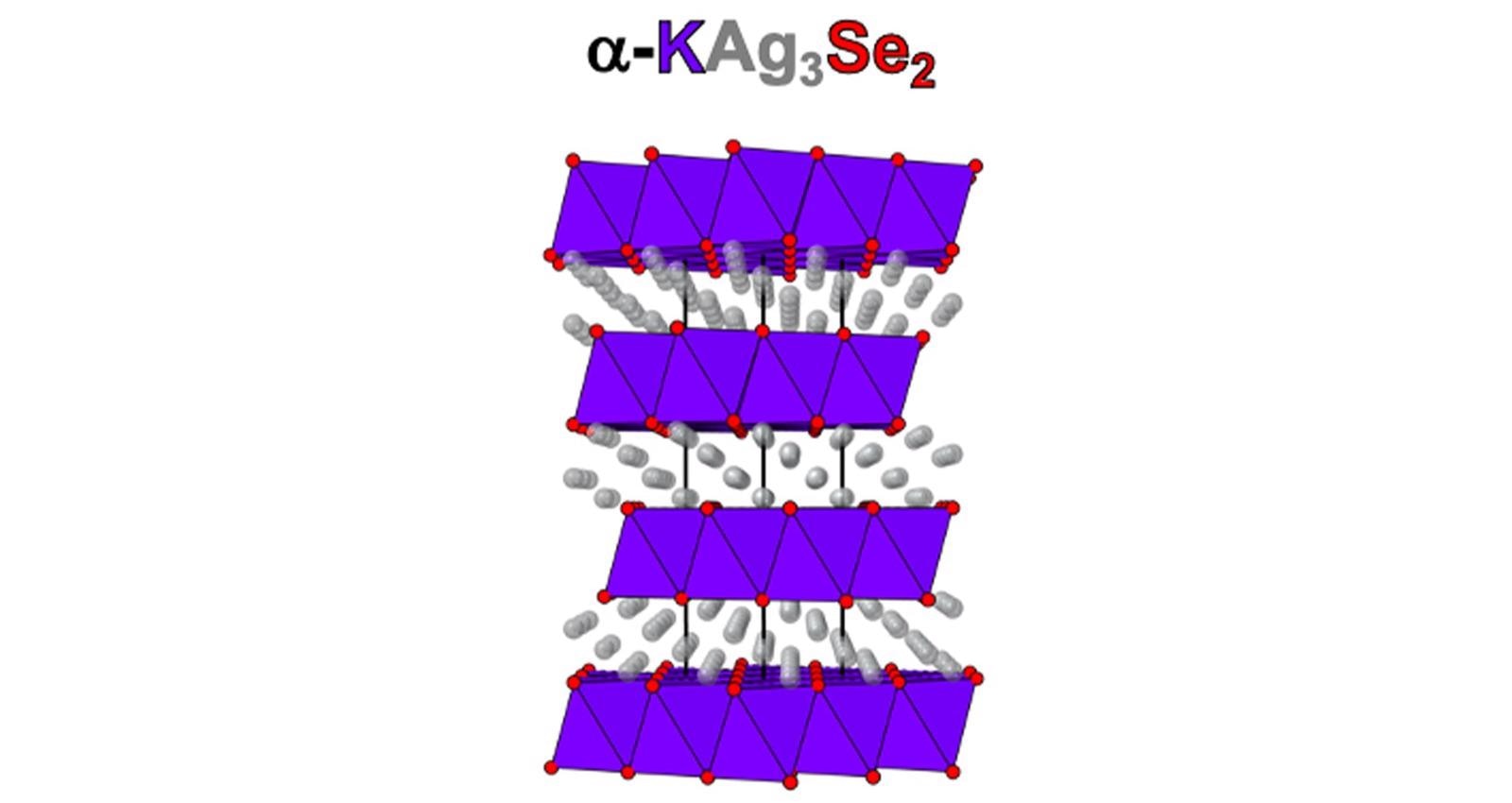 Four-layer atomic structure of α-KAg3Se2, a 2D superionic conductor. The colors of the atoms are coordinated with the colors in the name. Image Credit: Mercouri Kanatzidis/Northwestern University and Argonne National Laboratory.
Four-layer atomic structure of α-KAg3Se2, a 2D superionic conductor. The colors of the atoms are coordinated with the colors in the name. Image Credit: Mercouri Kanatzidis/Northwestern University and Argonne National Laboratory.
Mercouri Kanatzidis made an unanticipated discovery while he was hunting for a new superconductor featuring unconventional behavior. The discovery was a material measuring only four atoms in thickness and enabling the analysis of the motion of charged particles in just two dimensions. Studies like this could trigger the invention of new materials for a range of energy conversion devices.
Kanatzidis is not only a professor at Northwestern University but also has a joint appointment in the U.S. Department of Energy’s (DOE) Argonne National Laboratory. His target material was a blend of silver, potassium and selenium (α-KAg3Se2) in a four-layered configuration similar to that of a wedding cake. These novel two-dimensional (2D) materials have width and length, but virtually no thickness at just four atoms high.
When cooled to extremely low temperatures, superconducting materials tend to lose their resistance to the movement of electrons.
Much to my disappointment, this material was not a superconductor at all, and we could not make it one. But much to my surprise, it turned out to be a fantastic example of a superionic conductor.
Mercouri Kanatzidis, Senior Scientist, Materials Science Division, Argonne National Laboratory
In the case of superionic conductors, the charged ions in a solid material travel just as easily as in a battery’s liquid electrolytes. This leads to a solid with remarkably high ionic conductivity, which is a measure of the capacity to carry electricity.
During high ionic conductivity, low thermal conductivity tends to occur, which would prevent heat from passing through easily. Both of these properties render superionic conductors excellent materials for devices used to store and convert energy.
The researchers realized that they had found a material with extraordinary properties only when they heated it to between 450 ℉ and 600 ℉. It evolved into a highly symmetrical layered structure. They also discovered this evolution is reversible when they decreased the temperature and then increased it again to the high-temperature zone.
Our analysis results revealed that, before this transition, the silver ions were fixed in the confined space within the two dimensions of our material. But after this transition, they wiggled around.
Mercouri Kanatzidis, Senior Scientist, Materials Science Division, Argonne National Laboratory
Although a lot is known about how ions travel in three dimensions, there is very limited information about how they perform in only two dimensions.
For quite a while, researchers have been looking to find a prototypical material to explore ion movement in 2D materials. This unique layered potassium-silver-selenium material fits the bill. The researchers measured how the ions traveled in this solid and learned that it was comparable to that of a heavily salted water electrolyte, which is one of the fastest recognized ionic conductors.
It may be a bit early to tell if this specific superionic material might translate into a practical application. However, it could serve as a vital platform for engineering other 2D materials with low thermal conductivity and high ionic conductivity straightaway .
These properties are very important for those designing new two-dimensional solid electrolytes for batteries and fuel cells.
Duck Young Chung, Principal Materials Scientist, Materials Science Division, Argonne National Laboratory
Further research based on this superionic material could also lead to the development of new thermoelectrics that can change heat into electricity in industrial processes, power plants and even exhaust gas from vehicle emissions. Furthermore, such research could be used to create membranes for cleaning up the environment and for water desalination.
The research paper titled “A two-dimensional type I superionic conductor” has been published in the journal Nature Materials.
Besides Kanatzidis and Chung, other authors of the study include Alexander J. E. Rettie, Xiuquan Zhou, Michael J. Johnson, Jingxuan Ding, Christos D. Malliakas, Raymond Osborn, Olivier Delaire, Naresh C. Osti, and Stephan Rosenkranz. The team also included scientists from Argonne, University College London, DOE’s Oak Ridge National Laboratory, Northwestern, and Duke University.
For the experimental measurements, the team used the Spallation Neutron Source at Oak Ridge National Laboratory, beamline 17-BM-B at Argonne’s Advanced Photon Source, a DOE Office of Science User Facility, and the Integrated Molecular Structure Education and Research Center at Northwestern. For their computer simulations, the team used the computing resources offered on Bebop, a high-performance computing cluster at Argonne.
The DOE Office of Science, Office of Basic Energy Sciences largely supported this study.
Journal Reference:
Rettie, A. J. E., et al. (2021) A two-dimensional type I superionic conductor. Nature Materials. doi.org/10.1038/s41563-021-01053-9.