Recently, the use of photocatalysts to lessen carbon dioxide (CO2) has received plenty of attention. Researchers from Tokyo Tech have created a new osmium (Os) complex that is capable of absorbing a total wavelength range of visible light and serving as a panchromatic redox photosensitizer for CO2 reduction.
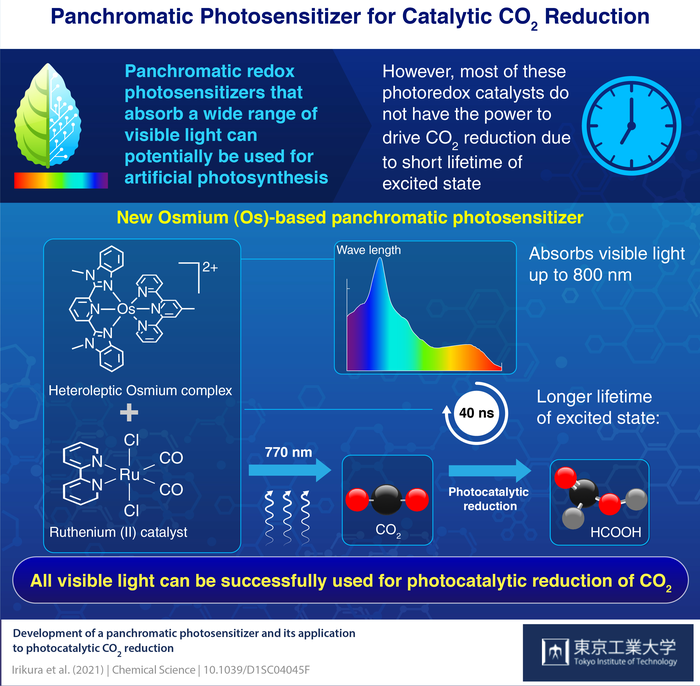
Image Credit: Osamu Ishitani, Tokyo Institute of Technology
The Tokyo Tech team integrated this complex with a ruthenium (II) catalyst and effectively reduced CO2 into formic acid.
Discovering solutions for the existing climate and energy crisis has become a communal goal worldwide. Why seek distant solutions when there is a perfect solution close by? Drawing inspiration from nature, researchers have been attempting to recreate the process of photosynthesis to fight climate change.
Other than helping plants make their food, photosynthesis also makes them one of the biggest carbon sinks that capture CO2 from the air and turn it into other forms. This makes artificial photosynthesis a profitable technique for not only water oxidation and hydrogen evolution but also for CO2 reduction.
The two main components needed to stimulate the multi-electron process of CO2 reduction are a redox photosensitizer that can absorb visible light and stimulate electron transfer, and a catalyst that can receive the electrons from the redox photosensitizer, trigger CO2 and finally present these electrons into CO2.
To best exploit solar energy, the photosensitizer has to be sensitive to a broad range of light wavelengths. Accordingly, panchromatic redox photosensitizers, materials that absorb the total wavelength of visible light, are the answer.
Ruthenium (Ru) complexes are the generally used redox photosensitizers that capture light and reach “excited” states through the metal-to-ligand charge transfer process. But, they cannot utilize low-energy parts of visible light as they cannot capture this light. The majority of reported panchromatic complexes cannot also be used for photoredox reactions as the lifetimes of their excited states are very short.
Scientists from the Tokyo Institute of Technology (Tokyo Tech) headed by Dr. Yusuke Tamaki and Prof. Osamu Ishitani tried out a new approach to enhance the photoredox properties of panchromatic photosensitizers. The researchers created a new Os complex that could absorb the total wavelength range of visible light. Details of their work have been published in Chemical Science, the Royal Society of Chemistry’s peer-reviewed flagship journal.
Employing this complex as the redox photosensitizer and a ruthenium complex catalyst (Ru(CO)), they created a photocatalytic system that could reduce CO2 into HCOOH (formic acid).
We were on the lookout for photocatalytic systems that allowed effective utilization of the solar light to carry out artificial photosynthesis. This is when we focused our attention to the photochemical properties of Os complexes, which rise from the heavy atom effect of Os. Since the photophysical, photochemical, and photosensitizing properties of Os complexes were not explored, we decided to test its abilities in CO2 reduction.
Osamu Ishitani, Professor, Tokyo Institute of Technology
The UV-visible absorption spectrum revealed that the Os complex absorbed visible wavelengths up to 800 nm, i.e., including red light. The complex showed a comparatively long excited-state lifetime of 40 ns, adequate for stimulating electron-transfer processes needed for reduction.
To perform the photochemical reduction experiments, the researchers irradiated the combined Os photosensitizer and Ru(CO) using 770 nm light. The system photocatalytically turned CO2 into formic acid with favorable reaction turnover numbers.
This research can be extended by using the panchromatic Os photosensitizer to perform other beneficial photochemical reactions such as organic photoredox reactions and H2 evolution from water.
The implications of our study are two-fold. Firstly, we demonstrated that all visible light can be used as energy for photocatalytic CO2 reduction. Secondly, we used the heavy atom effect to construct new redox photosensitizers that can absorb a wide range of visible light.
Osamu Ishitani, Professor, Tokyo Institute of Technology
Journal Reference:
Irikura, M., et al. (2021) Development of a panchromatic photosensitizer and its application to photocatalytic CO2 reduction. Chemical Science. doi.org/10.1039/D1SC04045F.