Acidophilic Extremophiles may sound like the kind of creature conjured up by the mind of H.R. Giger, but in reality, they are microorganisms that can live and thrive in acidic environments. Researchers at the Department of Earth Resources Engineering, Kyushu University in Japan have conducted the first study in relation to the effectiveness of these microorganisms as a tool in the production of platinum nanoparticles, or Pt(0)NPs, published in the journal Minerals.
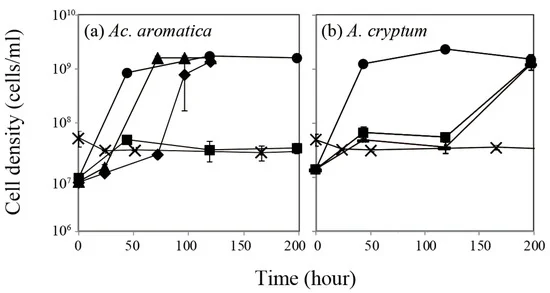 Effect of Pt(IV) on aerobic growth of Ac. aromatica (a) and A. cryptum (b) at pHinitial 2.5. Initial Pt(IV) concentrations were set to 0 mg/L (●), 0.5 mg/L (▲), 0.75 mg/L (◆), 1 mg/L (■), 2.5 mg/L (▬), or 5 mg/L (×). Data points are mean values from duplicate flasks. Image Credit: Matsumoto, T, Minerals.
Effect of Pt(IV) on aerobic growth of Ac. aromatica (a) and A. cryptum (b) at pHinitial 2.5. Initial Pt(IV) concentrations were set to 0 mg/L (●), 0.5 mg/L (▲), 0.75 mg/L (◆), 1 mg/L (■), 2.5 mg/L (▬), or 5 mg/L (×). Data points are mean values from duplicate flasks. Image Credit: Matsumoto, T, Minerals.
Professor Naoko Okibe, lead author and associate professor of Earth Resources Engineering at Kyushu University, states, “Extreme acidophiles, broadly defined as microorganisms that grow optimally at pH < 3, have potential in metal NPs’ production from such highly acidic liquors but are rarely studied in this regard.”
Typically, platinum nanoparticles are deployed as high-performance catalysts in automobiles, fuel cells, and petrochemicals, as well as having good application potential in medicine, optics, drug delivery, and electronics.
Furthermore, research investigating possible future applications of metal nanotechnologies is accelerating as a result of the developing technological innovations associated with them, which include alternative energy solutions.
Nanotechnology and Green Energy
Platinum nanoparticles could play a key role in “green” hydrogen production via water electrolysis as platinum is an exceptional reaction catalyst. This makes the research all the more interesting and vital as there is already a spotlight on alternative, renewable energies such as green hydrogen as well as hydrogen-based fuel cells for electric vehicles and in order to combat the climate crisis.
Green hydrogen is typically produced by splitting water using electricity generated from a low-carbon source. However, most of the hydrogen produced globally in 2020 was generated from fossil fuel sources with 99% of hydrogen fuel coming from carbon-based sources and is not considered green hydrogen.
Therefore, the application of nanotechnologies in processes in alternative energy sources could offer another way out of relying on carbon-based energy sources. However, this means that methods of producing metal nanoparticles, including platinum nanoparticles, should also aim to be environmentally friendly.
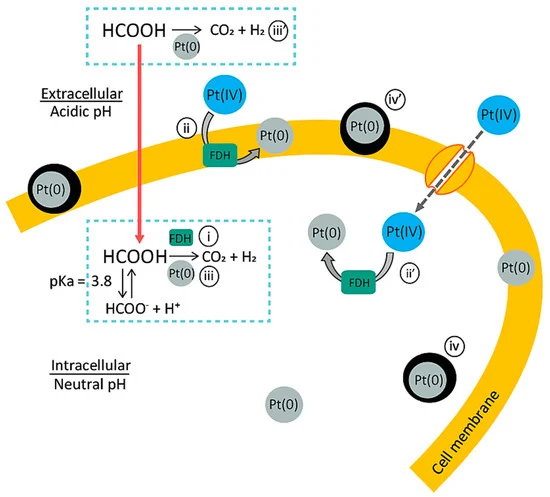
Proposed mechanism of the bio-Pt(0)NPs’ production in active cells: (i) Formic acid (HCOOH, pKa = 3.8) existing under the acidic condition can diffuse through the cell membrane. The putative formate dehydrogenase (FDH) enzyme catalyzes the decomposition of HCOOH to CO2 and H2 (i). Accordingly, at each FDH site on the cell membrane (ii) and in the cytosol (ii’), Pt(IV) ions are reduced by H2 to form the initial Pt(0) crystal nuclei (first slow reaction). Once Pt(0) nuclei are formed, Pt(0) starts to act as a chemical catalyst to accelerate the HCOOH decomposition reaction (iii). This autocatalytic reaction leads to the crystal growth of Pt(0)NPs (iv,iv’) (second, faster reaction). When the corresponding enzymes are (at least partially) deactivated by Cu2+, the number of crystal nucleation sites becomes limited, but the individual particle grows larger (the overall reaction time becomes shorter). Image Credit: Matsumoto, T, Minerals.
Platinum Nanoparticle Synthesis
However, despite growing demand and the importance of platinum, it is considered one of the rarer metals on Earth with only an average abundance factor of 5um/kg. Due to this scarcity, only a few hundred tons are produced each year and considering its application potential, it is extremely valuable and a major commodity.
Conventionally, the synthesis of platinum nanoparticles, and other metal nanoparticles, necessitates the use of top-down and bottom-up multi-step physical and chemical methods which may involve the use of toxic materials in hazardous environments.
This has led to a search for safer, greener alternatives, with the biological fabrication of metal nanoparticles attracting significant interest. “The microbiological route for Pt(0)NPs’ production is considered a greener and simpler alternative to conventional methods,” says Okibe.
Until recently, methods of biological fabrication of metal nanoparticles explored a range of organisms and life-forms including, algae, bacteria, fungi, plants, and yeast, with some of these demonstrating the ability to produce zero-valent nanometals.
However, one of the challenges that remain in place is that the metal-bearing solutions used for top-down methods of nanoparticle synthesis, or production, tend to be extremely acidic. While the organisms used express some tolerance towards acidic conditions, they are generally considered to be neutrophiles.
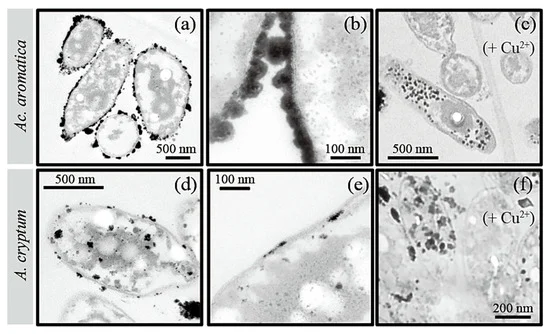
TEM images of bio-Pt(0)NPs produced by active cells of Ac. aromatica (using 20 mM of formate, (a–c)) or A. cryptum (using 10 mM of formate, (d–f)), without (a,b,d,e) or with (c,f) the addition of 5 mM of Cu2+ as a potential enzymatic inhibitor. Image Credit: Matsumoto, T, Minerals.
Acidic Extremophiles
This is where the untapped potential of acidic extremophiles could play a role as an important tool in metal nanoparticle synthesis, as they have the ability to survive and thrive in acidic conditions. “Acidophilic extremophiles often inhabit metal-rich acidic liquors in nature and are expected to become the promising tool for metal nanotechnology,” said Okibe.
The team used a highly acidic platinum solution for the study and used acidophilic Fe(III) iron-reducing bacteria using a simple one-step microbiological reaction streamlining the process. As a result, they witnessed the formation of well-dispersed bio-platinum nanoparticles. However, they discovered that the catalytic properties of the metal nanoparticles varied greatly depending on the presence of an enzymatic inhibitor in the process.
Overall, the study—the first of its kind—did show great promise, with Okibe stating, “Further studies on acidophilic extremophiles (especially with higher metal and salt tolerances) for biogenic metal NPs’ production would benefit the development of nanobiotechnology.”
This could pave the way for a more efficient and environmentally friendly way of producing platinum and other metal nanoparticles with good catalytic activity. This would in turn have a positive impact on other areas of research including “green” hydrogen production and hydrogen fuel cells.
References:
Matsumoto, T.; Phann, I.; Okibe, N. Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria. Minerals 2021, 11, 1175. https://doi.org/10.3390/min11111175
‘Fuel Cells’, energy.gov [https://www.energy.gov/eere/fuelcells/fuel-cells], accessed October 2021.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.