A certain amount of time must pass before a fuel cell may begin to operate and ensure a safe and extended useable lifetime. The current work analyzes the limitations of the reliability and accuracy of voltage measurement to determine the safe beginning point of operation in an attempt to integrate modeling and experimentation.
Fuel Cells Produces Energy
Fuel cells are categorized by the kind of electrolyte used, as well as the starting time, which may range from 1 second to 10 minutes. Batteries are a comparable technique in which the fuel may be renewed by recharging. A fuel cell produces electricity using the energy stored as hydrogen or any other fuels in a clean and efficient manner.

G60 test station (left) and zoom-in on the single-cell compression unit (right). Image Credit: Bodner, M., et al, Energies
Only water, electricity, and heat are produced when hydrogen is used as a fuel. Fuel cells are distinctive in that they are using a wide range of sources and feedstocks or biofuels, so they can power generation as large as a municipal power plant and as tiny as a personal laptop.
The Innovations on Operational Techniques
The corresponding innovations are in the midst of becoming introduced to the market, thanks to a slew of international and regional financing programs. Yet, fuel cells in real-world applications still have a long way to go in terms of endurance. Furthermore, operational techniques are critical for increasing system resilience.
While a solid operating strategy can provide a stable, dependable, and lengthy lifespan, a badly designed approach can result in essential components dying early and unexpectedly. Start-up and shut-down are two crucial processes in the operation of a fuel cell.
Consequently, the existence of air-hydrogen before and during the start-stop operation is a recognized trigger for significant cathode deterioration, and alternate solutions have been tried; not all of them have been effective, and some have even caused harm. The deployment of a load after reactant gases have been introduced is another crucial phase in any operating plan.
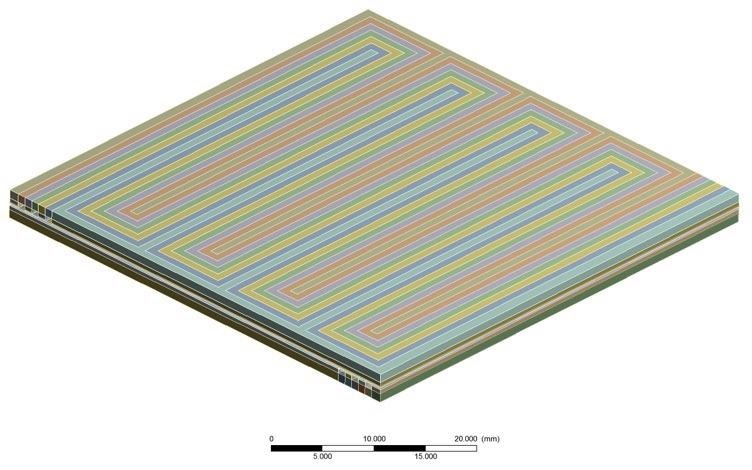
Decomposed geometry of the anode and cathode flow fields. Image Credit: Bodner, M., et al, Energies
Results Show Time Reduced
The method has resulted in a reduction in the time required to perform such computations by several orders of magnitude; for example, a total simulation duration of 75 seconds lasts 3 hours when using a 1s step, but it would take 156 days with a 1ms step. This was achieved by employing a high quality of mesh, a mist flow hypothesis, and operating circumstances that allow for such a method, namely moderately humidified reagents and initial concentration.
A mixture of processes is most likely responsible for a tiny amount of carbon corrosion. Remnants of oxygen in test unit anode pipework, particularly from oxygen cross-over from cathode to the anode, can react with hydrogen when it is injected, due to variations in heat at the catalyst active locations.
The oxidized carbon is enhanced by higher temperatures, the placement of a catalyst, and the water content, as evidenced by CO2 emissions. There is continuously reduced hydrogen content at the cell's exit as compared to when the cells are traversed and when analyzed.
Membrane Resistance
For membrane resistance, the concept relies on a single parameter. In fact, this is the limitation because it will not be stable throughout the entire range of current operating points, and so the inaccuracy is most likely due to the membrane resistance input variable. Because the simulation tends to overestimate the concentration of the hydrogen, concentrations at the intake and outflow have rather substantial errors at the start of the experiment.
Further Research with Polymer Electrolyte
The research points to the fact that there has been no fuel starvation. The construction of hydrogen-powered automobiles of this quality in the transportation sector seeks to enhance fuel economy and greatly minimize engine emission and intensity. Although in minimal amounts, the measured CO2 emissions seem to be related to the entrance of the reaction mixture rather than a local hydrogen shortage.
The generated mathematical analysis will be utilized in future research to investigate the performance of PEM fuel cells using conventional driving cycles, with the findings compared to current density distributions obtained locally.
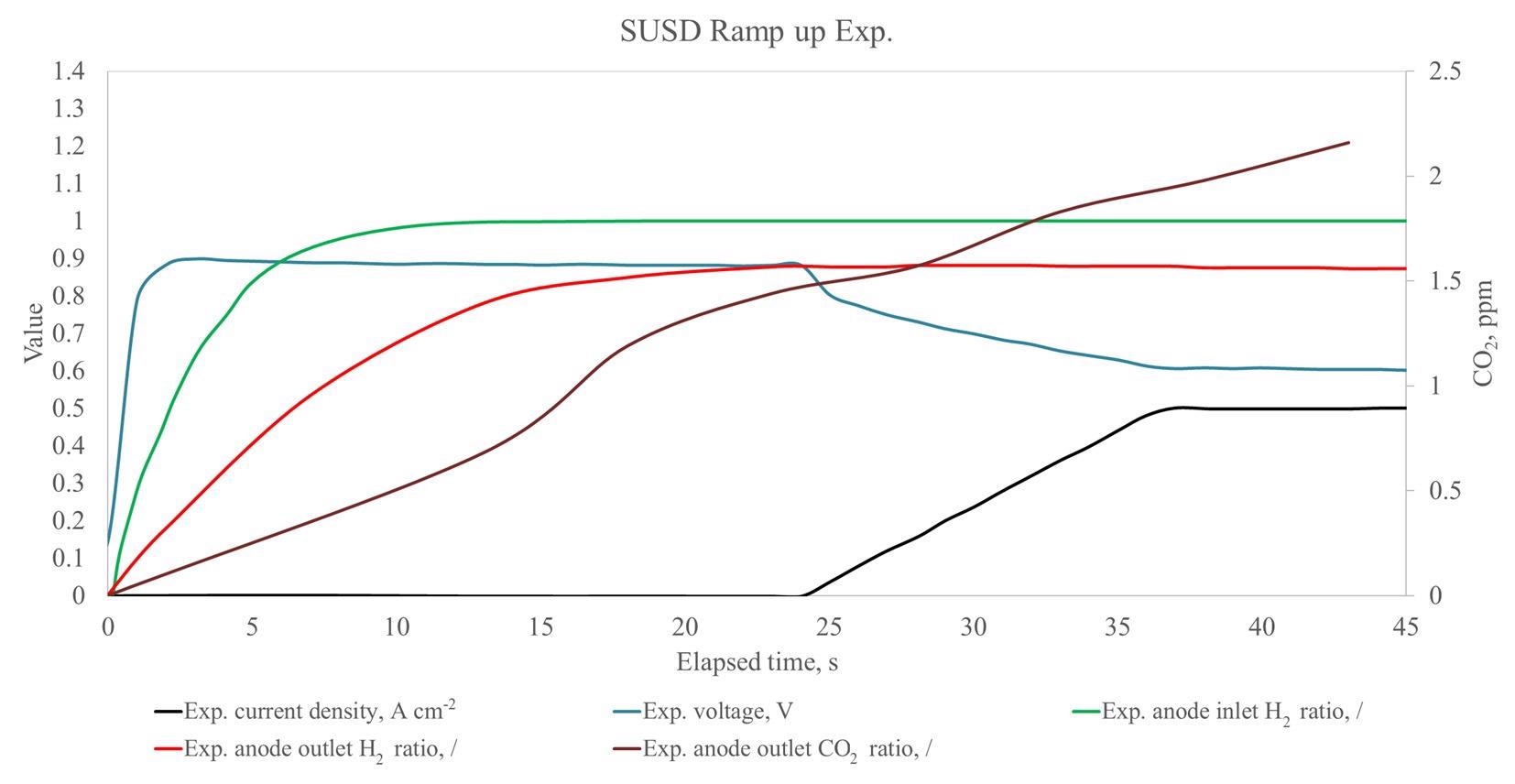
Measured gas compositions during start-up. Image Credit: Bodner, M., et al, Energies
Considering the significance of hydrogen energies as clean and renewable fuels in internal combustion, it is crucial to look at how they affect compression and spark-ignition engines. The simulations show that the reduced time in starting machines can help in future development.
References
Bodner, M., et al. (2021). Simulation-Assisted Determination of the Start-Up Time of a Polymer Electrolyte Fuel Cell. Energies, 14(23), 7929; https://www.mdpi.com/1996-1073/14/23/7929
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.