The erratic supply of green electricity necessitates large-scale storage to maintain the stability of the power grids. Since standard batteries are not good for scaling, the concept of using flow batteries that store energy in a liquid is appealing.
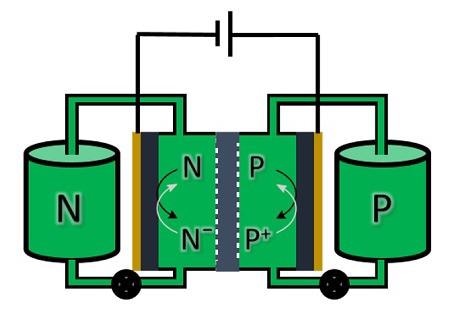 Schematic representation of a redox flow battery. The electrolyte solutions are pumped from storage tanks through an electrochemical cell where charge/discharge takes place. Image Credit: E. Otten, University of Groningen
Schematic representation of a redox flow battery. The electrolyte solutions are pumped from storage tanks through an electrochemical cell where charge/discharge takes place. Image Credit: E. Otten, University of Groningen
However, these batteries comprise rare metals and are costly. Researchers at the University of Groningen, the Netherlands, have built a flow battery electrolyte that may resolve both issues. Their findings have been reported in the Journal of the American Chemical Society on 8th March.
Flow batteries are not so different from the batteries that people use on a daily basis. The main difference is that the energy is kept in two individual liquids with dissolved chemicals for charge storage.
Electricity is stored (and later discharged) by pumping these fluids via an electrochemical cell that comprises a membrane via which ions can be swapped. The energy content of this type of battery is scalable by just using bigger storage containers for the fluids.
Expensive
In recent times, China deployed flow batteries to decrease the inconsistency in green electricity production.
Large-scale storage capacity is needed when intermittent sources, such as solar and wind energy, become more prominent in the electricity mix, because the grid might get destabilized. The type of battery that the Chinese use was designed in the1980s and is based on a solution containing vanadium.
Edwin Otten, Associate Professor of Molecular Inorganic Chemistry, University of Groningen
This metal is extracted only in a few regions on Earth. “This means that the supply cannot always be guaranteed and it is rather expensive,” Otten explains.
Moreover, it necessitates a particular membrane to divide the two fluids, which also increases the costs. That is why Otten’s research team, along with colleagues from the University of Eindhoven (the Netherlands) and the Technical University of Denmark, aimed to develop a new type of flow battery material.
Blatter Radical
We wanted a symmetrical battery where both tanks contain the same fluid. Also, we wanted it to be based on an organic molecule rather than on a metal.
Edwin Otten, Associate Professor of Molecular Inorganic Chemistry, University of Groningen
The two sides of the flow battery usually hold fluids with a diverse composition. Symmetrical batteries have been engineered by connecting the molecules that are found on both sides and filling both containers with the ensuing hybrid molecule.
“The drawback of this approach is that only one part of the molecule is used on either side. And, during use, reactive radicals appear that degrade over time. This makes stability a problem.”
Otten and his colleagues applied a different method. They hunted for a single stable molecule that could donate or accept electrons and could, thus, be employed on either side of the battery. The most favorable compound turned out to be a Blatter radical, a bipolar organic compound that can either donate or accept an electron in a redox reaction.
“The molecule that we selected was also intrinsically stable,” says Otten.
They verified the compound in a small electrochemical cell. It functioned well and stayed stable across 275 charge/discharge cycles.
“We need to bring this up to thousands of cycles; however, our experiments are a proof of concept. It is possible to make a symmetrical flow battery that has good stability.”
The organic Blatter radical is comparatively easy to manufacture and although it is presently not made in industry, scale-up can be done.
Imbalance
Another advantage of our symmetrical design is that it is not a big problem if some of our compound crosses the membrane during use. This could result in a slightly higher volume in one of the tanks but any imbalance is easily restored by simply reversing the polarity.
Edwin Otten, Associate Professor of Molecular Inorganic Chemistry, University of Groningen
During their experiments, the team demonstrated that this indeed functions as projected. Other experimental projects of symmetrical batteries were not sufficiently stable to achieve the number of cycles required to prove this.
The subsequent step is to develop a water-soluble variety of the Blatter radicals. The majority of flow cells are developed for water-based fluids since water is inexpensive and not combustible.
“PhD students in my group are already working on this.” An additional step is to increase the solubility and stability of the Blatter radical and verify it on a larger scale.
The crucial test is to see whether our compounds will be stable enough for commercial applications.
Edwin Otten, Associate Professor of Molecular Inorganic Chemistry, University of Groningen
Journal Reference:
Steen, J. S., et al. (2022) Blatter Radicals as Bipolar Materials for Symmetrical Redox-Flow Batteries. Journal of the American Chemical Society. doi.org/10.1021/jacs.1c13543.