For grid storage applications, there is a pressing demand for safe, low-cost, and dependable high-capacity batteries. Non-aqueous redox flow batteries (RFBs) allow for greater voltages outside of the water voltage stability window and are compatible with alkali metal anodes with ultrahigh energy density.
Redox-targeting is an approach for increasing RFB solubility that uses soluble redox-active molecules (redox mediators, RMs) to oxidize and reduce solid energy-storing materials. It is believed that by combining the unique concepts from the Li-metal anode and RFB communities, a high-performing mediated LiS RFB can be created.
Lithium-sulfur provides battery chemistry that is appealing due to its high energy density and cheap sulfur cost. For reasons of safety, scalability, and affordability, scaling up to the MWh needed for grid-scale energy storage necessitates a new strategy.
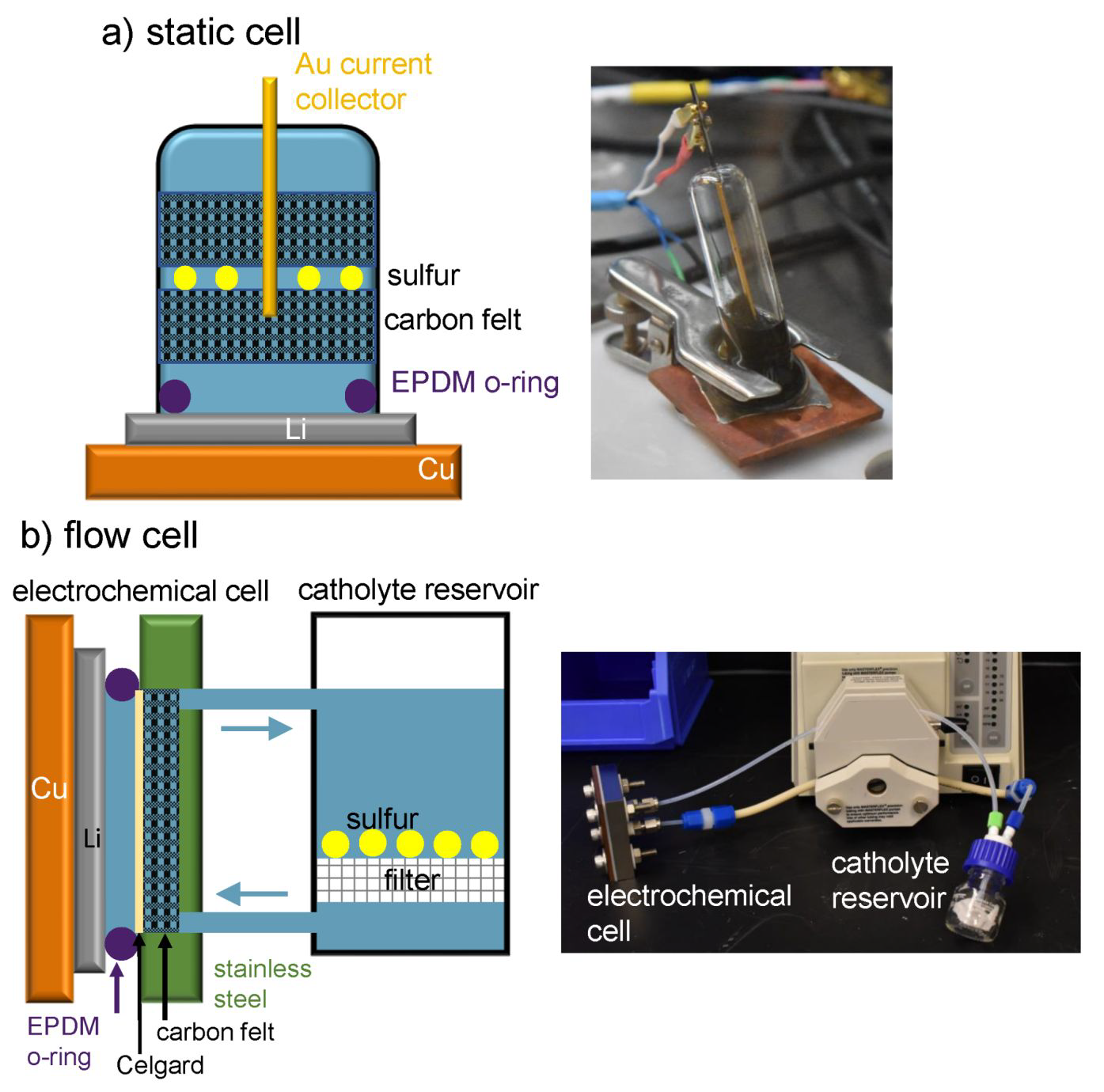
a) Static cell design, b) flow cell design. Image Credit: Meyerson, M. L. et al., ACS Applied Energy Materials
About the Study
In this study, the authors presented a redox-targeting approach and its integration with the designed Li solid electrolyte interphase (SEI) to create a high-efficiency, scalable, membrane-less LiS redox flow battery. The Li anode was contained in the electrochemical cell in this hybrid flow battery architecture, while the solid sulfur was kept safe in a different catholyte reservoir, and the electrolyte was pushed over the sulfur and into the electrochemical cell.
The team used decamethylferrocene (DmFc) and cobaltocene (CoCp2) as redox mediators owing to their electrochemical simplicity and capability to initiate the corresponding initial reduction of solid S into the soluble polysulfides and the final reduction of polysulfides into the solid Li2S without the use of conductive carbons.
The researchers used electrochemical and spectroscopic approaches to verify the efficacy of the proposed processes in static cells. The LiS chemistry from static cells, redox mediation, and a flow cell design were combined to effectively adapt the LiS chemistry for use in a mediated and membrane-less RFB.
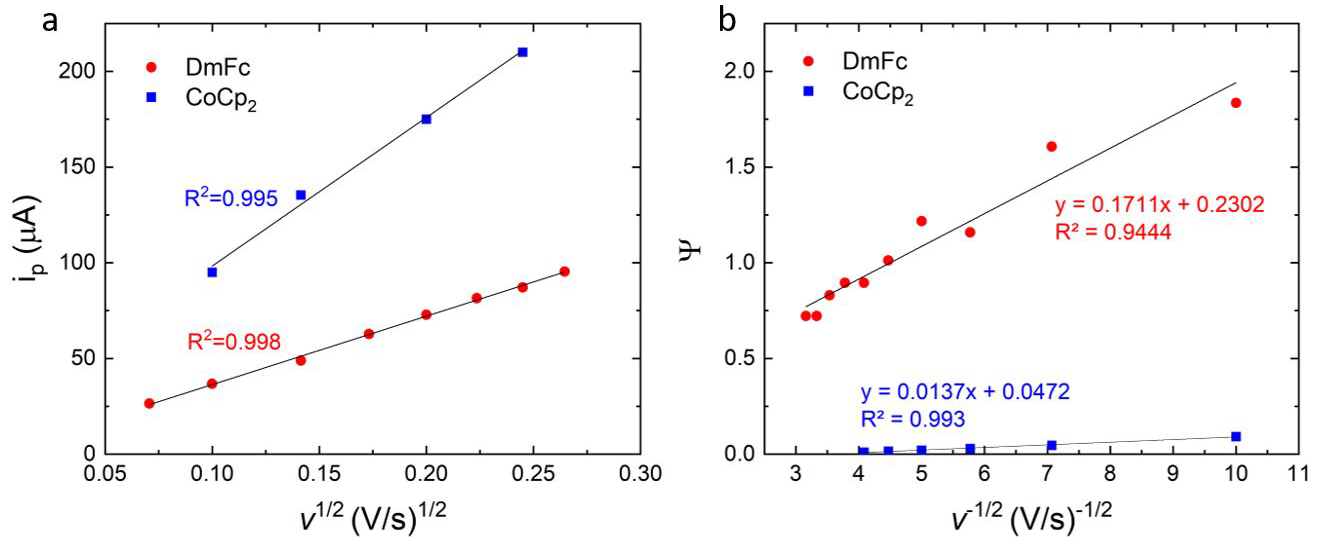
a) Peak current and b) ψ vs square root of the scan rate for cyclic voltammograms of 8.3 mM decamethyl ferrocene and 20 mM cobaltocene in 1 M LiTFISI in 1:1 vol% DOL:DME. Glassy carbon working electrode, Li reference electrode, and Pt counter electrode. Image Credit: Meyerson, M. L. et al., ACS Applied Energy Materials
Observations
In the absence of any ion-selective separators, a LiI and LiNO3 pre-treatment method on the Li anode resulted in a stable SEI and reduced capacity fading. At 1-2 mgS cm-2 (973 mAh gS-1 and 81.3% voltage efficiency (VE) in static cells and 1142 mAh gS-1 and 86.9% VE in the flow cells), equivalent areal loadings of up to 50 mgS cm-2 (84 mAh cm-2) were observed. Following that, over 2-50 mgS cm-2 was demonstrated in a lab-scale membrane-less flow cell where particles were actively filtered and discharge periods surpassed 66 hours.
A LiI and LiNO3 pre-treatment method on the anode side promoted a stable SEI, reduced capacity fading, and eliminated the need for ion-selective separators. The materials analysis validated the homogeneous distribution of LiI in the corresponding SEI, whereas scanning electron microscopy (SEM) analysis indicated the presence of reduced surface area globular deposition of Li, and ultraviolet-visible (UV-vis) spectroscopy confirmed the evolution of polysulfide species. The Li anode's consumption of PS and S species was found to be responsible for capacity fading.
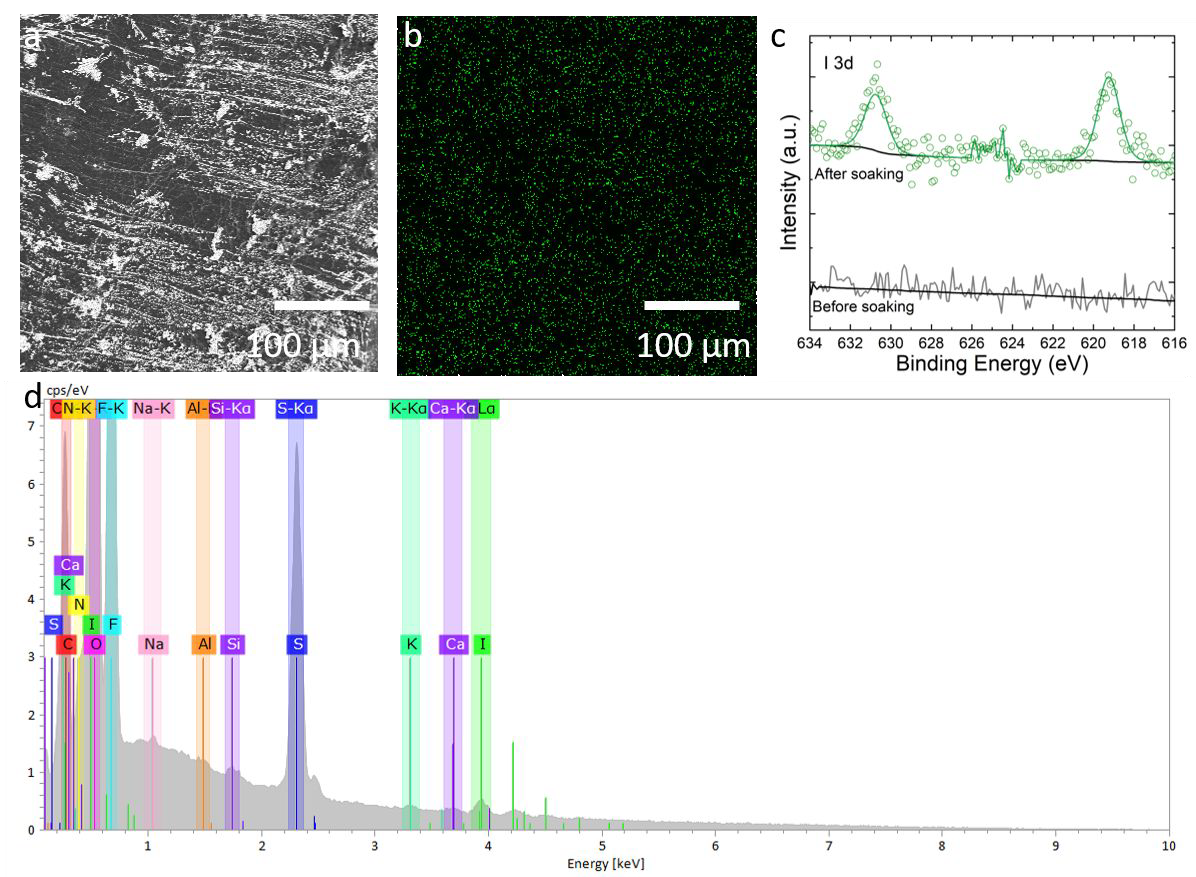
a) SEM of Li surface after soaking in 20 mM LiI overnight, b) EDX map of I corresponding to area in a, c) XPS of I 3d region, I 3d5/2 at 619.2 eV corresponds to I in the form of LiI, and d) EDX spectrum of a. Image Credit: Meyerson, M. L. et al., ACS Applied Energy Materials
According to the results, the core LiS chemistry and innovative SEI engineering methodologies could be transferred to the architecture of the hybrid redox flow battery. Although a continuous increment in the S loadings was required for this chemistry to reach its full potential, such loadings necessitated lengthy cycling durations at the lab scale. With improvements to the cell architecture and Li anode, the performance characteristics of these cells could be improved even more. This could be accomplished by increasing flow field uniformity, which would increase contact between the catholyte and the carbon counter electrode and encouraged more uniform deposition of Li on the Li anode.
Conclusions
In conclusion, this study elucidated the development of suitable redox mediators (RMs) for commercially available S and paired them with a stabilized Li SEI via LiI pre-treatment and a LiNO3-containing electrolyte.
The authors emphasized that using a 3D scaffold to increase the effective surface area corresponding to the Li anode should improve the homogeneity of the Li deposition while also allowing cycling at quicker charge rates and testing of even greater S loadings.
They believe that the fundamental SEI engineering strategies and LiS chemistry can be applied to the architecture of the hybrid redox flow battery, which can eliminate the need for flowing carbon additives or ion-selective membranes and could pave the way for low-cost, scalable, and safe MWh-scale LiS energy storage. They also mentioned that the performance assessment shown in this study points to a bright future for grid-scale energy storage that is safe, energy-dense, and scalable.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.