Textile wastewaters contain a wide range of colors and chemicals that produce major environmental issues that have an impact on human health and aquatic life. As a result, effective treatment is required prior to emission. Photocatalysis is widely regarded as one of the most effective methods for detoxifying organic-contaminated wastewater. As a very efficient photocatalyst, TiO2 has a few key flaws that have limited its use on a global scale so far. To address these issues, several researchers began adding TiO2 to the body of carbon compounds in order to create photocatalytic materials.
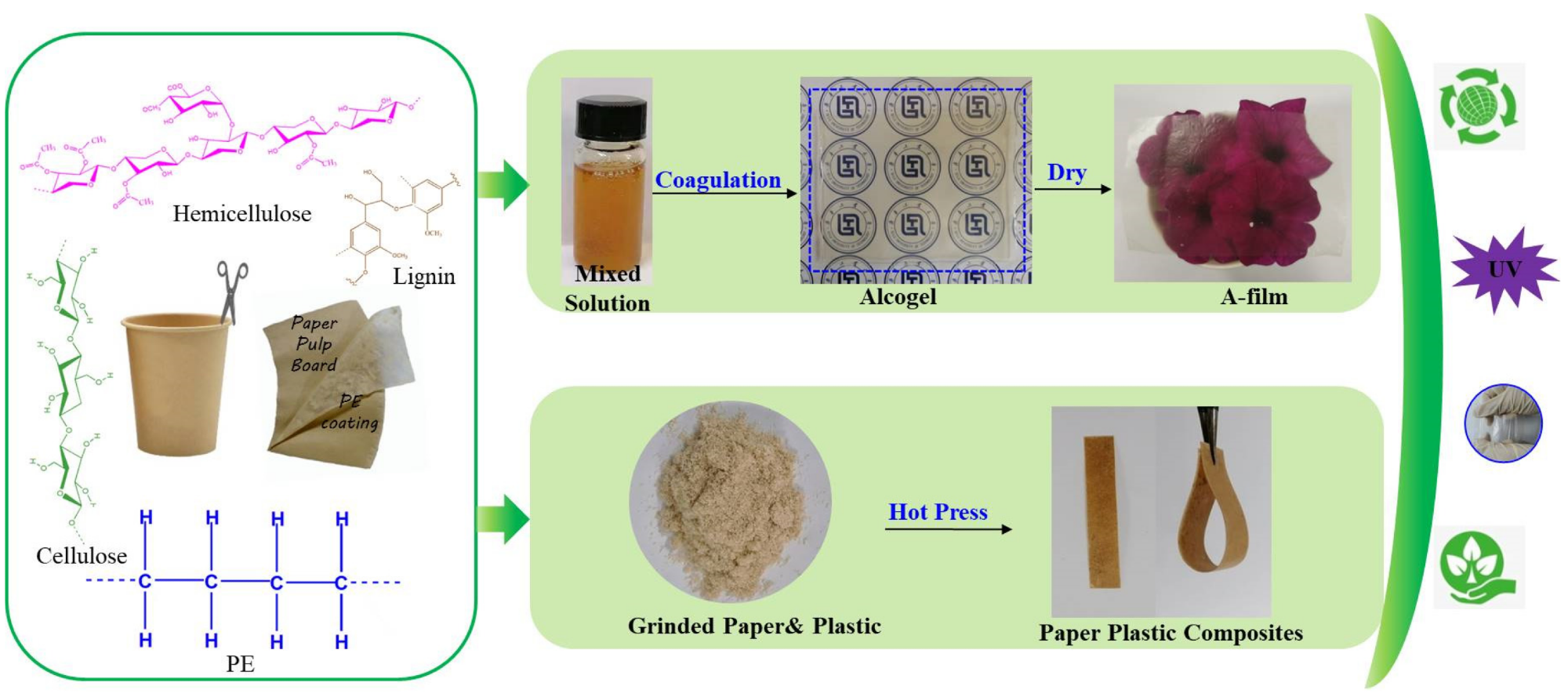
Process of converting waste disposable paper cups (WDPC) into cellulose-based films (H-film and A-film) and paper-plastic composites (PPC) by AmimCl and hot press methods. Image Credit: Chen, Y et al., Polymers
Biomass-derived carbon materials have received a lot of attention in the field of carbon materials. Rabbit hair fiber with a hollow structure, in particular, is a sustainable protein biomass material that, following carbonization, might be an ideal transporter. The biocarbon fiber enhances the separation efficiency of photogenerated electron-hole pairs and the performance characteristics of the photocatalytic degradation of organic pollutants by using the catalyst. In the realm of photocatalysis, recycling these waste rabbit hairs is critical, and making proper use of them will maximize potential value.
About the Study
In this study, the authors presented the synthesis of nanocomposites of TiO2/carbonized waste rabbit fibers (TiO2/CRFs) with hierarchical microporous/mesoporous structures by utilizing waste rabbit hair as the carbon source and tetrabutyl titanate as the titanium source, plus a combination of immersion, carbonization, and calcination processes. Several characterization techniques and methylene blue (MB) photodegradation investigations were used to assess the catalytic activity and characteristics of the TiO2/CRFs composite.
The researchers presented the experimental basis for the use of waste rabbit biomass carbon composites in photocatalytic degradation. By using a simple impregnation and calcination procedure, biomass protein carbon fibers, i.e., carbonized waste rabbit hair fibers, were used as a carrier for TiO2 and nitrogen and carbon-doped TiO2/CRFs composites with a hierarchical structure in the photocatalytic degradation of the methylene blue.
The photocatalytic efficacy of TiO2/CRFs composites for methylene blue degradation was studied under varying TiO2:CRFs mass ratios, reaction times, photocatalyst dosages, and methylene blue initial concentrations. Cycle experiments were used to assess the possibility of material reuse and recovery.
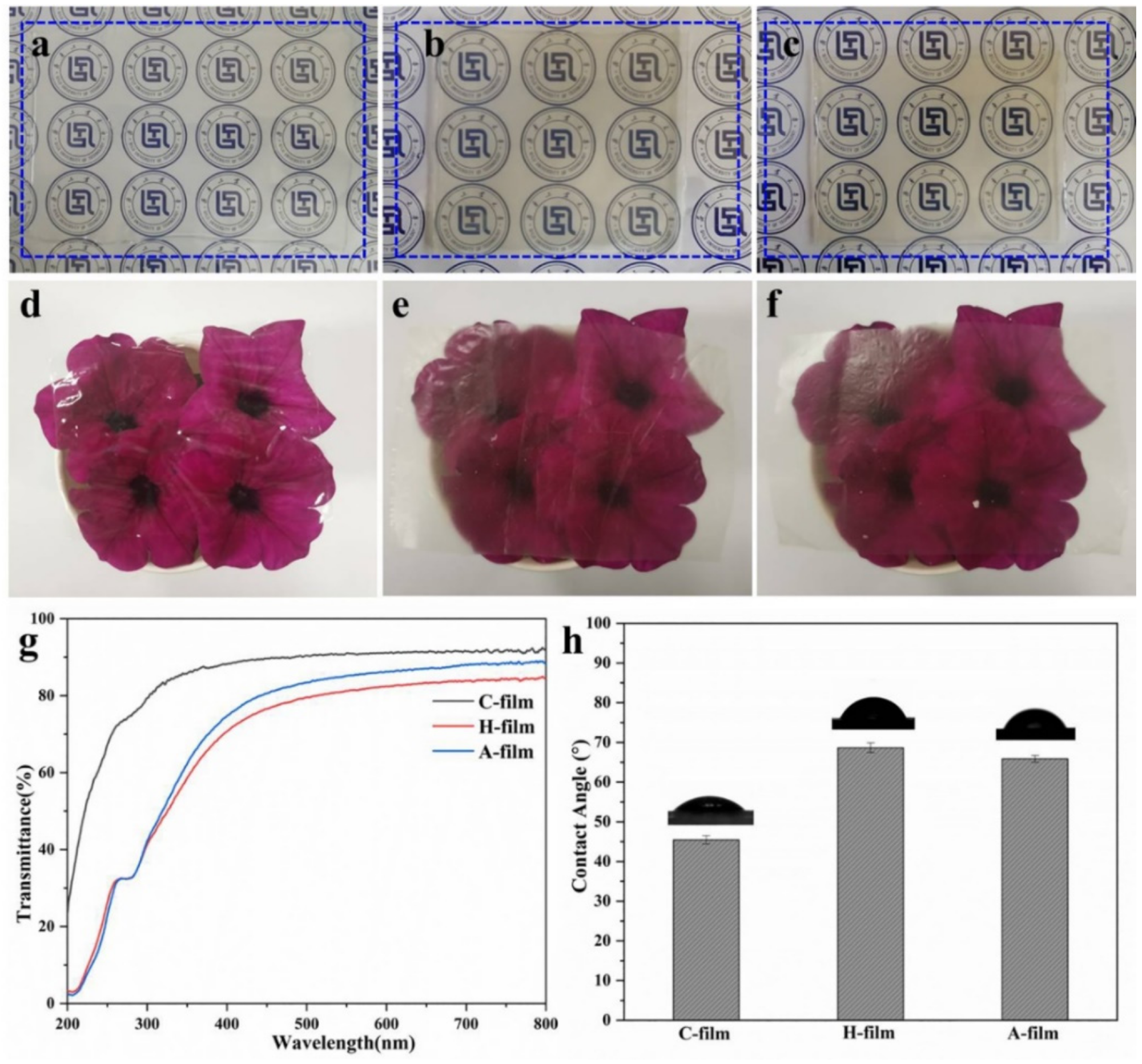
Optical pictures of the regenerated gels (a) C-gel; (b) Hydrogel; (c) Alcogel) and films (d) C-film; (e) H-film; (f) A-film; (g) UV-Vis spectra of C-film, H-film and A-film; (h) Water contact angles of the C-film, H-film, and A-film. Image Credit: Chen, Y et al., Polymers
The team demonstrated the development of a novel TiO2/CRFs nanocomposite photocatalyst with increased photocatalytic performance and high stability by combining carbonization, impregnation, and calcination methods. TiO2/CRFs were naturally doped with carbon and nitrogen elements derived from waste rabbit hair, which effectively reduced the compounding of photogenerated electron-hole pairs and the bandgap of TiO2, increased the absorption of visible light, and improved TiO2's photocatalytic activity.
Observations
The results showed that after 80 minutes of sun irradiation, the degradation of MB by the TiO2/CRFs reached 98.1%. After five cycles of degradation tests, TiO2/CRFs maintained excellent photocatalytic activity and demonstrated good reusability and stability. The improved photocatalytic characteristics of TiO2/CRFs materials were attributed to the natural nitrogen and carbon element doping of TiO2/CRFs materials, as well as their morphology, which reduced the constituent of photogenerated electron-hole pairs and narrowed the TiO2 band-gap.
On the other hand, the multiple reflections of the visible light in pore channels enhanced the materials' visible light absorption. Furthermore, the huge specific surface area ensured that adsorbed reactants had plenty of reaction sites.
Because the produced material had a large specific surface area, the anatase titanium dioxide nanoparticles were uniformly filled on the scale structure of CRFs, improved the dye's adsorption capacity, provided more reaction sites, and solved the problem of problematic TiO2 recycling.
When compared to the first cycle, the degradation efficiency of TiO2/CRFs of MB was lowered by 3.9%. The ideal amount of TiO2/CRFs photocatalyst in the photocatalytic experiment was 1.25 g L-1, and the degradation rate reached 97.9%. When the UV lamp was irradiated for 60 minutes, the degradation rate of MB solution was only 85.35% when compared to bare TiO2. After 60 minutes of irradiation, the degradation rates of the samples were 98.0% at 5 mg L-1 MB, 97.9% at 10 mg L-1 MB, 88.8% at 15 mg L-1 MB, and 50.9% at 20 mg L-1 MB. The degradation rates of MB solution were 97.9%, 41.9%, and 98.3% when TiO2/CRFs dosages were 1.25 g L-1, 0.41 g L-1, and 1.46 g L-1, respectively.
At an initial concentration of 10 mg/L, a TiO2/CRFs ratio of 5:1, and a material dosage of 1.25 g/L, the photocatalytic degradation of MB solution by TiO2/CRFs could reach 98.1% after 80 minutes of solar irradiation.
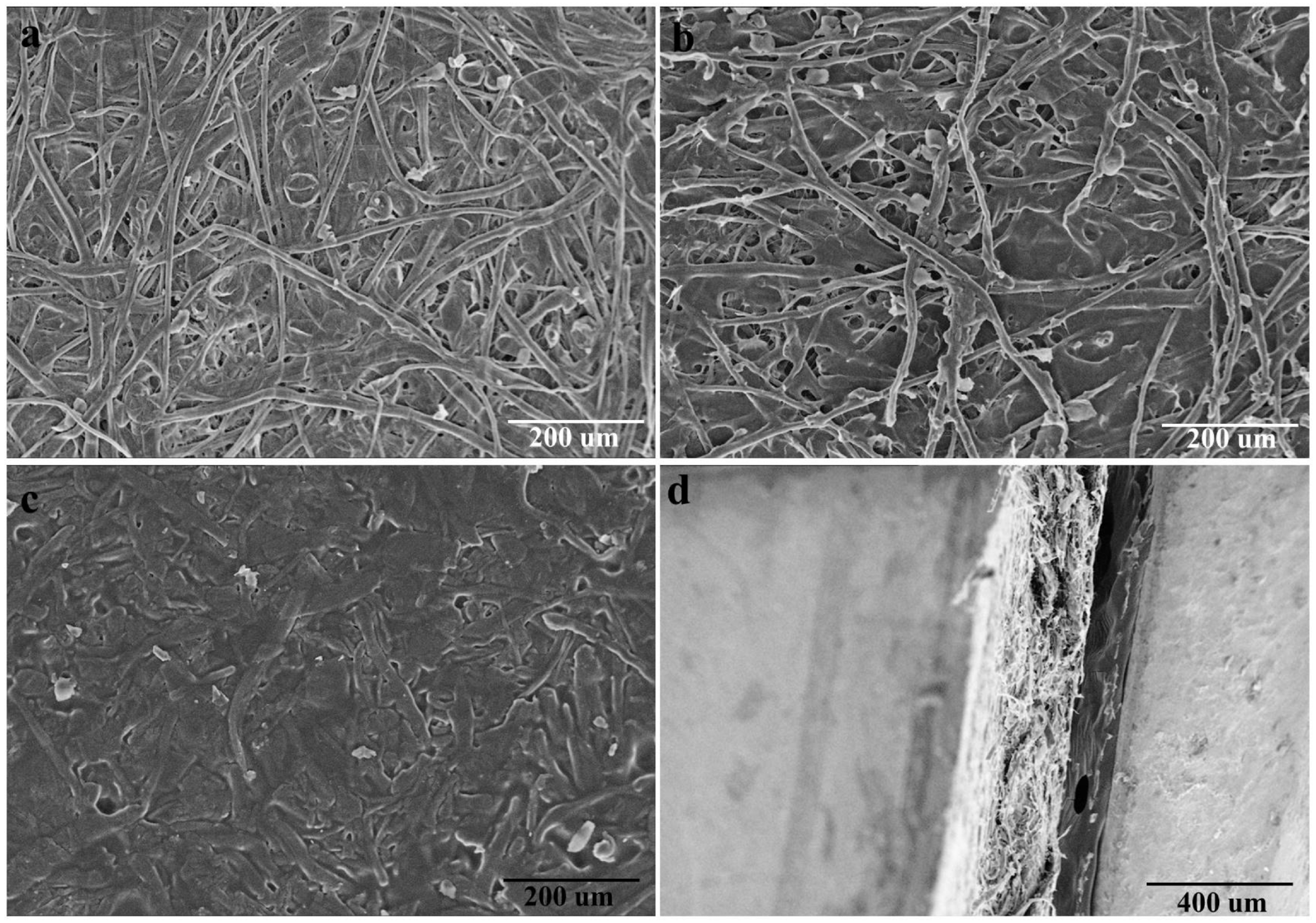
(a,b) The SEM images of waste disposable paper cups (WDPC) inner surface before and after peeling off the thin PE coating; (c,d) SEM images (c), the surface and (d), the cross section) of the paper plastic composites (PPC). Image Credit: Chen, Y et al., Polymers
Conclusions
In conclusion, this study elucidated that the TiO2/CRFs composites play a key role in lowering the band-gap width due to natural nitrogen and carbon doping.
The authors emphasized that its huge specific surface area and unique hierarchical micropore/mesopore structure provide an abundance of active sites for reactant adsorption, while the pore structure's numerous reflections improve the material's light-trapping ability.
They also believe that given the low cost and waste reuse of rabbit hair, as well as the non-toxic and strong photocatalytic activity of TiO2, this work presents an economical and environmentally friendly technique for manufacturing biochar/TiO2 composite photocatalysts.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.