Using energy and natural resource conservation to start sustainable manufacturing processes is one of the main objectives of the circular economy. For communities with low landfill capacities and ineffective municipal waste management (MWM) systems, garbage disposal and emission have grown to be significant concerns.
Glass is an inorganic material that has been vitrified and is generally recovered from municipal solid waste (MSW) at recycling plants. The only type of shattered glass that can be recycled to make new clear glass containers is clear broken glass, also known as cullets. However, it is an expensive and time-consuming operation to separate clear glass from colored glass.
Micropollutant-induced water contamination has increased over the past few decades, endangering both the environment and human growth. With the goal of replacing activated carbons with a sustainable material created using a circular economy approach, a wide range of alternative low-cost and ecologically friendly adsorbents have been investigated. For applications in water treatment, relatively little study has been done with inorganic wastes like glass and glass ceramics. Only a few studies have looked into using silica glass frit in place of sand in rapid and slow sand filters. Even fewer have used synthetic glasses or post-processed waste glasses for wastewater treatment, despite the fact that the aforementioned applications used glasses without structural alteration or post-processing.
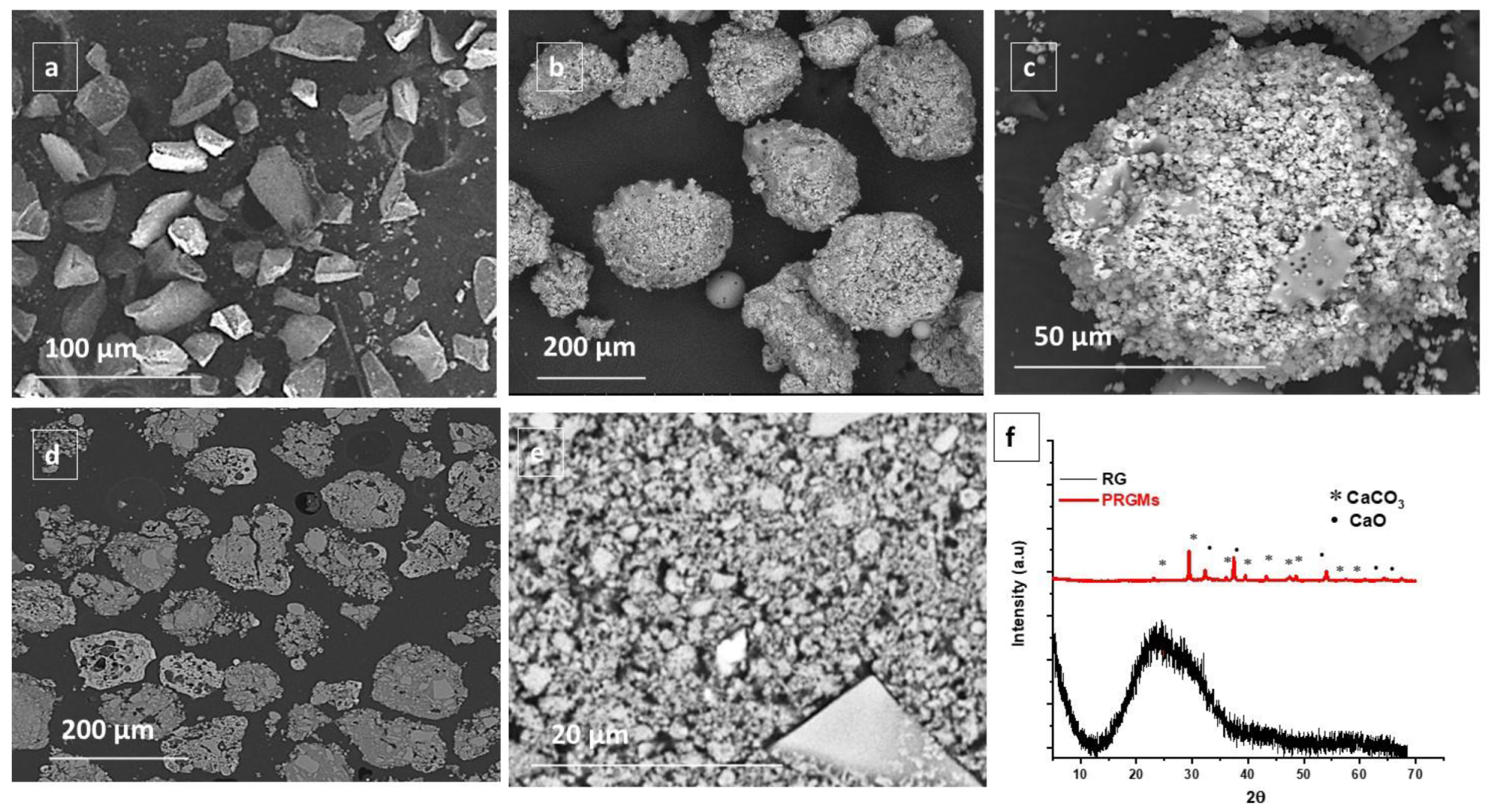 SEM images of (a) as received RG (particle size ≤ 63 µm), (b,c) surface morphology of porous recycled glass microspheres (PRGMs) at different magnification, (d,e) cross-section of PRGMs at different magnification and (f) XRD pattern of RG and PRGMs. Image Credit: Samad, S. A et al., Materials
SEM images of (a) as received RG (particle size ≤ 63 µm), (b,c) surface morphology of porous recycled glass microspheres (PRGMs) at different magnification, (d,e) cross-section of PRGMs at different magnification and (f) XRD pattern of RG and PRGMs. Image Credit: Samad, S. A et al., Materials
About the Study
In this study, the authors devised a solvent-free upcycling technology for recycled glass trash (RG) into porous and recycled glass microspheres (PRGMs) in order to investigate the removal of organic contaminants such as organic dyes. A flame spheroidization procedure was used to create PRGMs, and they were then characterized using scanning electron microscopy (SEM), Brunauer-Emmett-Teller (BET), X-ray diffraction (XRD), and mercury intrusion porosimetry (MIP) analyses.
With a total pore area and pore volume of 8.6 cm2/g and 0.84 cm3/g, respectively, and a surface area value of 8 m2/g, PRGMs demonstrated 69% porosity. As model sources of contaminants, methylene blue (MB) and acid red 88 (AR88) were investigated. The results demonstrated that the PRGM dosages, pH of the dye solution, and dye concentrations had an impact on the removal of AR88 and MB by PRGMs. In the batch process trials, the removal of the AR88 dye was attributed to both adsorption and coagulation processes; however, only the adsorption process was responsible for the removal of the MB dye. For MB and AR88, the highest monolayer adsorption capacities (qe) were 20 mg/g and 78 mg/g, respectively.
The team demonstrated that hydrogen bond formation and electrostatic interaction were responsible for the adsorption process. For trials involving column adsorption, the PRGMs' ability to remove dye was also examined. At flow rates of 0.5 mL/min and 2.2 mL/min, respectively, the predicted adsorption capacities were 231 mg/g and 250 mg/g. The greater adsorption capabilities seen in the column adsorption tests were because of a synergistic interaction between adsorption/coagulation and filtration processes.
The researchers showed how to recycle or reuse RG particles for potential use as a pre-screen material to filter out pollutants in wastewater treatment. Through the use of the flame spheroidization (FS) procedure, RG particles were transformed into PRGMs, which were subsequently tested for their ability to remove the anionic AR88 and cationic MB dyes from water. In order to reuse, reduce, and up-cycle inorganic waste materials, this study focused on the elimination of the usage of toxic and dangerous chemicals and provided a straightforward processing route for the manufacture of RG into porous microspheres using the proposed FS technique.
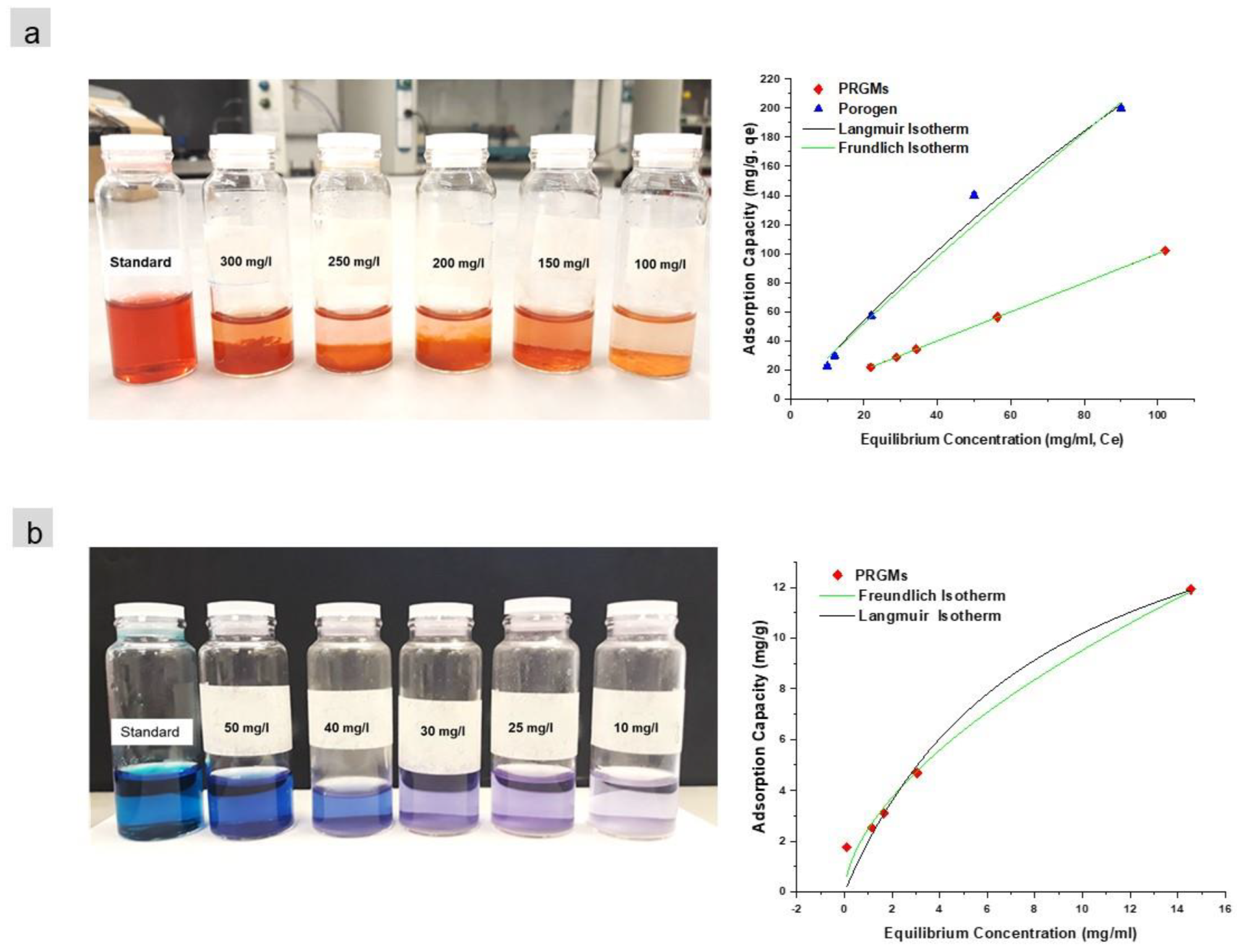
Isotherm data fitted with Langmuir and Freundlich models for (a) AR88 and (b) MB dye. (PRGMs dose = 5 g/L, pH 2.5 and pH 10 for AR88 and MB respectively and temperature of 22 ± 2 °C equilibrium time 24 h). Image Credit: Samad, S. A et al., Materials
Observations
For MB and AR88 dye, respectively, the ideal PRGMs doses were 6 g/L and 10 g/L, with pH values of 2.5 and 7, respectively. For MB and AR88 dye, the maximal monolayer adsorption capacities (qm) estimated for UW-PRGMs were 20 mg/g and 78 mg/g. The adsorption capacity increased with flow rate and was 305 mg/g for W-PRGMs, 381 mg/g for PRGMs, and 42 mg/g for RG, respectively. The ground-breaking Yoon-Nelson and Thomas-Bohart adsorption models suggested that a lower EBCT and higher flow rate could have an impact on the adsorption of AR88 onto PRGMs. The experimental data and the computed data, however, differed significantly.
The fifth cycle of the adsorption-desorption process was repeated in order to examine the PRGMs' reusability. After being heated for one hour at 400 °C in the furnace, the PRGMs that had been loaded with dye could be effectively regenerated. Following desorption, derivative thermogravimetric (DTG) analysis proved that the MB and AR88 dye had entirely broken down by 350 °C. For the second through fifth adsorption/desorption cycles, PRGMs demonstrated good recovery with nearly constant efficiency of 86–87%.
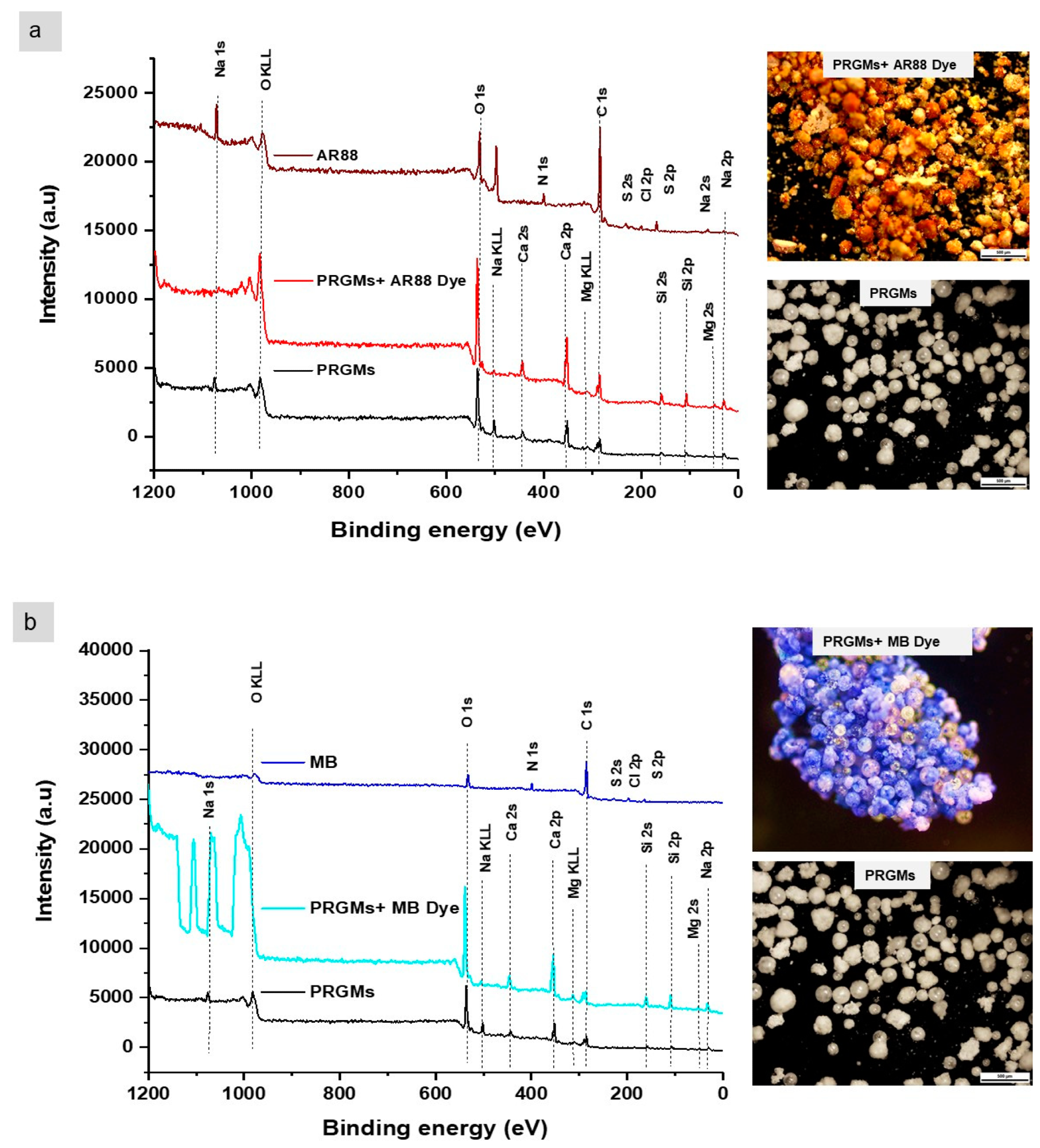
An XPS survey scan and optical microscopy images for PRGMs before and after (a) AR88 and (b) MB dye adsorption. Image Credit: Samad, S. A et al., Materials
Conclusions
In conclusion, this study discussed the production of PRGMs from RG using the flame spheroidization technique, and its suitability for water treatment applications was assessed. The adsorption kinetics and effectiveness of the PRGMs were affected by a number of variables, including adsorbent dosages, pH, and dye concentrations. For the purpose of removing the AR88 dye, a column adsorption study was carried out on W-PRGMs, RG, and PRGMs.
The authors focused on the reuse, recovery, and recycling of waste RG as well as how it could be used subsequently to filter out micro-pollutants for the treatment of wastewater, such as pre-screening materials. It was shown that PRGMs made from recycled glass debris could be used in the next cyclic experiment with equal success at removing dye.
The team mentioned that along with maximizing the useful life of discarded glasses by promoting reuse, recycling, and recovery, upcycling used glasses for other uses would also reduce the consumption of natural resources in the production of glass.
References
Samad, S. A., Arafat, A., Lester, E., et al. Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal. Materials, 15(17), 5809 (2022). https://www.mdpi.com/1996-1944/15/17/5809
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.