As the demand for electric vehicles increases, so does the need for high-safety, long-life batteries. However, the requirement for high-energy-density batteries in electric vehicles exceeds the capability of conventional lithium-ion batteries.
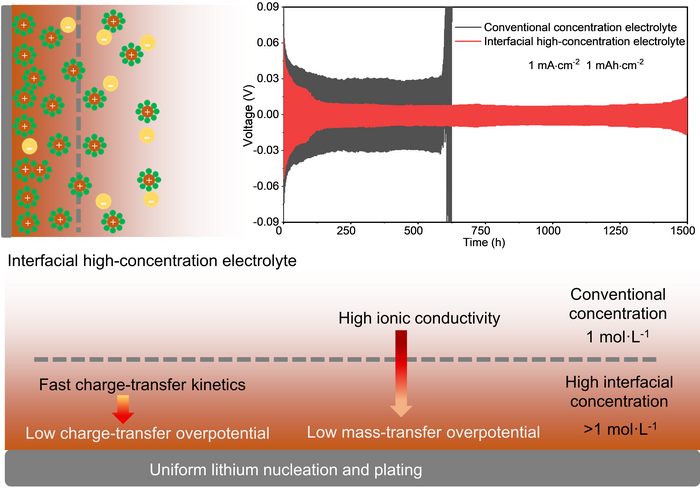 Schematic of the ion distribution of interfacial high-concentration electrolyte (upper left) and the discharge/charge voltage profiles of the conventional concentration electrolyte and the interfacial high-concentration electrolyte (upper right). The schematic at the bottom of the image illustrates the effect of the interfacial high-concentration electrolyte on lithium nucleation and plating. Image Credit: Nano Research, Tsinghua University Press.
Schematic of the ion distribution of interfacial high-concentration electrolyte (upper left) and the discharge/charge voltage profiles of the conventional concentration electrolyte and the interfacial high-concentration electrolyte (upper right). The schematic at the bottom of the image illustrates the effect of the interfacial high-concentration electrolyte on lithium nucleation and plating. Image Credit: Nano Research, Tsinghua University Press.
Lithium metal batteries offer a significantly larger charging capacity. Researchers are working to build lithium metal batteries utilizing lithium metal as the anode. However, there are safety concerns with lithium-metal batteries due to the formation of dendrites (spiky, metallic microstructures) during the charging process.
A group of Chinese investigators set out to overcome the problem of lithium dendrite formation and to develop high-safety, long-life lithium metal batteries. The team has effectively developed an electrolyte that prevents dendrite formation. This electrolyte performs well in lithium metal batteries and provides solutions for developing high-safety, long-life lithium metal batteries.
On October 3rd, 2022, the study was published in the journal Nano Research.
High-energy storage batteries can benefit significantly from lithium metal anodes; however, the unregulated growth of lithium dendrites creates serious issues. Dendrite formation occurs when lithium ions move and change at a particular spot on the lithium metal surface. Dendrites seriously compromise battery safety and reduce cycling efficiency.
The group solved the dendrite problem by integrating the benefits of traditional electrolytes and high-concentration electrolytes. High-concentration electrolytes show great promise for usage in next-generation batteries as they improve on some of the drawbacks of current electrolytes. The team’s new electrolyte inhibits dendrite formation while providing good electrochemical performance in lithium metal batteries.
Its unique structure not only promotes the uniform conversion of ions on the electrode surface but also ensures the rapid movement of ions in the electrolyte.
Chunpeng Yang, Professor, Tianjin University
The scientists started by running numerical simulations to investigate the influence of a negatively charged covering on the induction of the interfacial high-concentration electrolyte. The team then created the electrode by coating nitrogen- and oxygen-doped carbon nanosheets with nickel foam as a proof-of-concept material.
The positively charged lithium ions are concentrated near the nickel-coated nitrogen- and oxygen-doped carbon electrode. This concentration of lithium ions increases charge transfer reactions on the electrode, contributing to exceptional electrochemical cycling performance. The half-cell and full-cell testing on the electrode yielded outstanding results. Their electrode outperforms previous electrodes based on pure nickel foam.
This provides a simple principle for suppressing the lithium dendrites by simultaneously taking into account the advantages of conventional electrolyte and high-concentration electrolyte for stable Li metal anode, which may be applied to other substrates for practical metal batteries.
Chunpeng Yang, Professor, Tianjin University
The group intends to explore more strategies to create this special electrolyte structure to produce high-performance batteries in addition to coating negatively surface-charged materials on the electrode to direct the production of interfacial high-concentrated electrolytes.
By improving the battery components, the investigators seek to realize the commercial application of Li metal batteries with high energy density, high safety, and long life.
Our study results could be extended to more metal-battery systems, such as sodium, zinc, and magnesium metal batteries, which will contribute to the realization of large-scale energy storage for sustainable energy supply.
Chunpeng Yang, Professor, Tianjin University
The research team includes Haotian Lu, Feifei Wang, and Lu Wang from Tianjin University, the Haihe Laboratory of Sustainable Chemical Transformations, and the National University of Singapore.
Chunpeng Yang, from Tianjin University and the Haihe Laboratory of Sustainable Chemical Transformations; Jinghong Zhou, from East China University of Science and Technology; Wei Chen, from the National University of Singapore; and Quan-Hong Yang from Tianjin University and the Haihe Laboratory of Sustainable Chemical Transformations also participated in the study.
The National Key Research and Development Program of China, the Haihe Laboratory of Sustainable Chemical Transformations, and the Fundamental Research Funds for the Central Universities funded the study.
Journal Reference
Lu, H., et al. (2022) Interfacial high-concentration electrolyte for stable lithium metal anode: Theory, design, and demonstration. Nano Research. doi.org/10.1007/s12274-022-5018-7.