The development of sustainable batteries has been prompted by the significant demands for the transition to a low-carbon economy. Modern Li-ion batteries (LIBs) are unable to meet these criteria. Exploiting new architectures built on sustainable and abundant organic electrode materials (OEMs) and creating green and sustainable battery technologies beyond LIBs are essential for addressing this problem. Rechargeable potassium batteries (RPBs) stand out as a possible substitute.
As well as having poor energy and environmental sustainability, commercial LIBs are unable to meet the requirements for high-temperature and fast-charging applications. For energy storage devices to be used extensively, fast-charging, high-temperature RPBs must be developed as replacements for LIBs. OEMs can be used to get fast-charging, high-performance, and high-temperature RPBs. All-organic RPBs are excellent high-temperature RPB candidates. Due to OEMs' high flexibility, structural integrity is maintained even during rapid ion/electron transfer at high temperatures.
Rechargeable batteries could malfunction owing to the volatile organic solvent gassing in commercial electrolytes and the instability of solid-electrolyte interphase (SEI) at high operating temperatures. Therefore, creating cutting-edge OEMs and organic electrolytes is essential for RPBs that can quickly charge and operate at high temperatures.
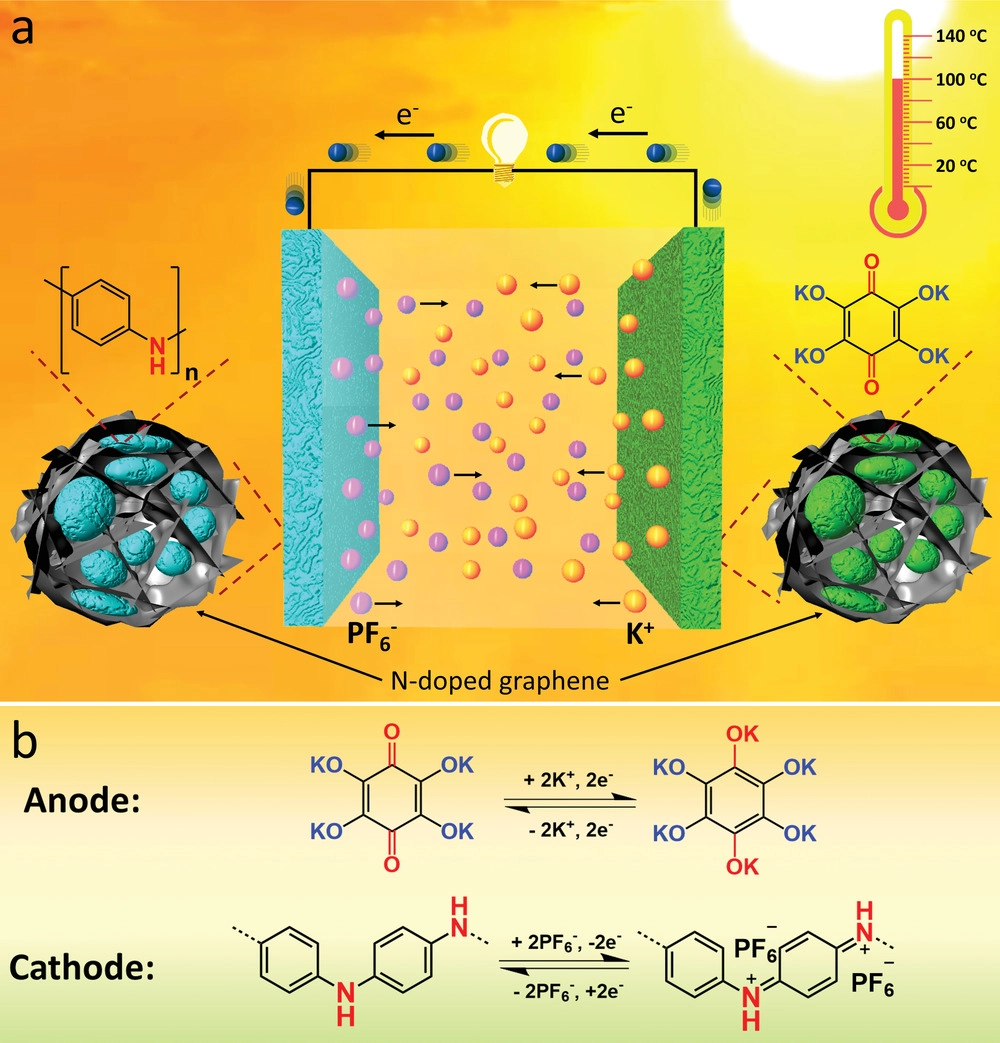
Schematic illustration and redox reactions in the all-organic battery. a) The schematic of the all-organic rechargeable potassium battery; b) Redox reactions in the organic anode and cathode during charge/discharge. Image Credit: Qin, K et al., Advanced Science
About the Study
In this study, the authors discussed the development of a fast-charging and high-temperature RPB without any transition metals. It was made of abundant and sustainable OEMs and potassium resources. To reduce OEM dissolution, accommodate significant volume changes, improve electrode conductivity, and create stable solid electrolyte interphase in the all-organic RPB, N-doped graphene (NG) and a 2.8 m potassium hexafluorophosphate (KPF6) in diethylene glycol dimethyl ether (DEGDME) electrolyte were used.
The team demonstrated that RPB exhibited an extremely stable and quick-charging all-organic battery at room temperature, delivered a high specific capacity of 188.1 mAh g-1 at 50 mA g-1 and exceptional cycle life of 6000 and 50000 cycles at 1 and 5 A g-1, respectively. The high-mass-loading all-organic RPB demonstrated high-rate capability up to 2 A g-1 and a long lifetime of 500 cycles at 70-100 °C. To boost conductivity, accommodate the significant volume change, and maintain the structural integrity of organic electrodes, the highly conductive NG was employed in both the cathode and the anode of the all-organic PRB.
The researchers illustrated that due to the low volatility of DEGDME and high boiling point of 162 °C, as well as the production of a stable K2CO3 and KF-rich SEI, a highly concentrated electrolyte called 2.8 m KPF6 in DEGDME, was utilized to further enhance the performance of the all-organic RPB. For the high-temperature and fast-charging all-organic RPB to remain stable, a stable electrolyte and SEI were crucial. The all-organic RPB performed admirably electrochemically at high temperatures of up to 100 oC and fast charging rates of up to 10 A g-1. The all-organic RPB's exceptional performance under adverse conditions was made possible by the reversible redox reactions between the amine- and carbonyl-based anodes and cathodes.
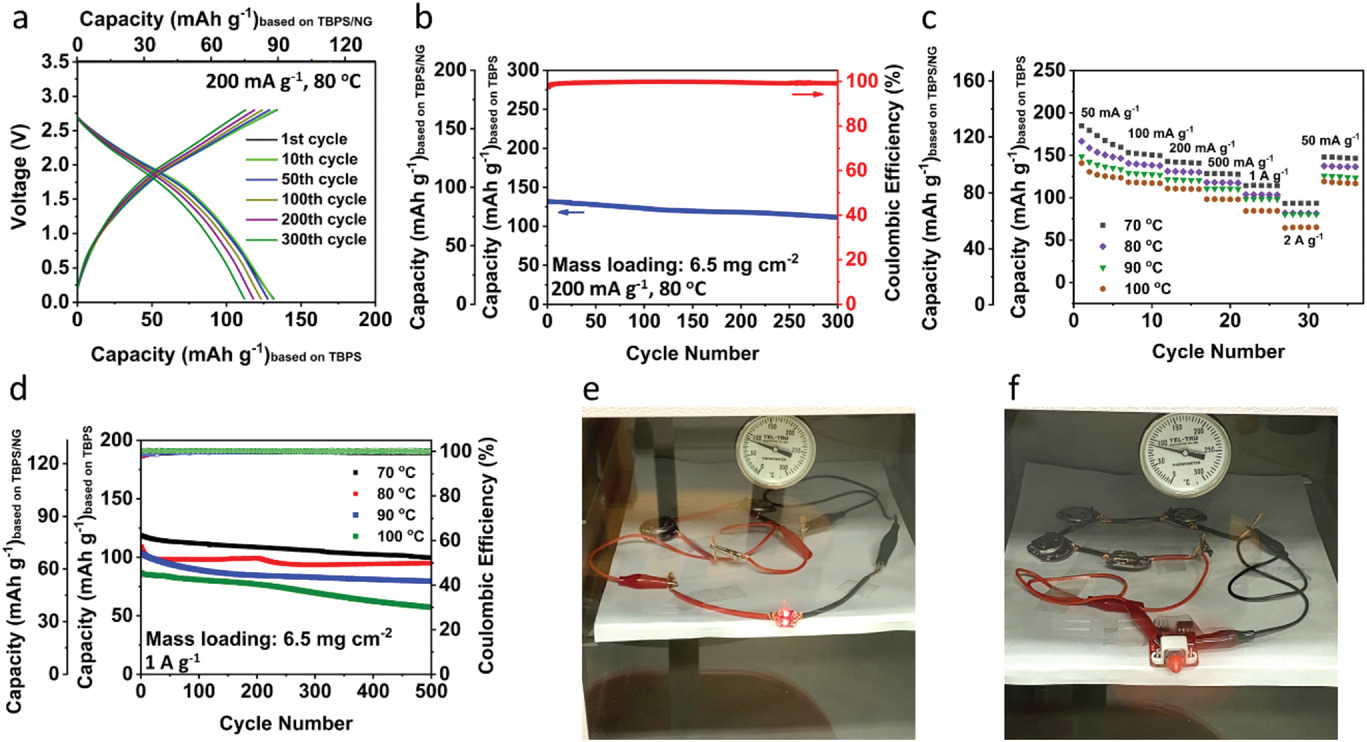
The electrochemical performance of the all-organic RPB with high mass loading at high temperatures. a) Selected charge/discharge curves and b) cycling stability of the all-organic RPB at 80 °C under the current density of 200 mA g−1. c) Rate capability of the all-organic RPB at high temperatures. d) Cycling stability of the all-organic RPB at high temperatures under the current density of 1 A g−1. e) Two red LED lights and f) a small fan powered by all-organic RPBs at ≈90 °C. Image Credit: Qin, K et al., Advanced Science
Observations
From 70 to 100 °C, the strength of the PO43- peak remained constant. The findings showed that the cathode electrolyte interphase (CEI) was made up of phosphate, organic fluorides, and KF, all of which display exceptional stability even at temperatures as high as 100 °C. The polyaniline (PANI)/NG cathode exhibited significantly more structural integrity than the tetrahydroxy-1,4-benzoquinone potassium salt (TBPS)/NG anode.
The TBPS/NG particles continued to be micro-sized structures at 100 °C and exhibited no evident cracks, but the surface was rough and small holes were produced, which indicated a less stable composite structure and SEI layer at this temperature. Additionally, after prolonged cycling, the all-organic RPB's average CE at 1 A g-1 and 80 °C was 99.8%. A reversible capacity of 99.9 mAh g-1 could still be maintained after 500 cycles. The all-organic RPB demonstrated capacity retention of 77.13%, 87.08%, and 66.28% for 500 cycles at 90, 80, and 100 °C, respectively. The all-organic RPB provided a high specific capacity value of 188.1 mAh g-1 at 50 mA g-1 and room temperature at low mass loading of 1.8 mg cm-2.
Superior cycle lifetimes of 50000 and 6000 cycles were attained, at the high current densities of 5 and 1 A g-1, respectively. At high temperatures and a high mass loading value of 6.5 mg cm-2, outstanding electrochemical performance was maintained. The all-organic RPB showed excellent fast-charging and high-temperature battery performance with quick charging up to 2 A g-1 and a long lifetime at 70-100 °C.
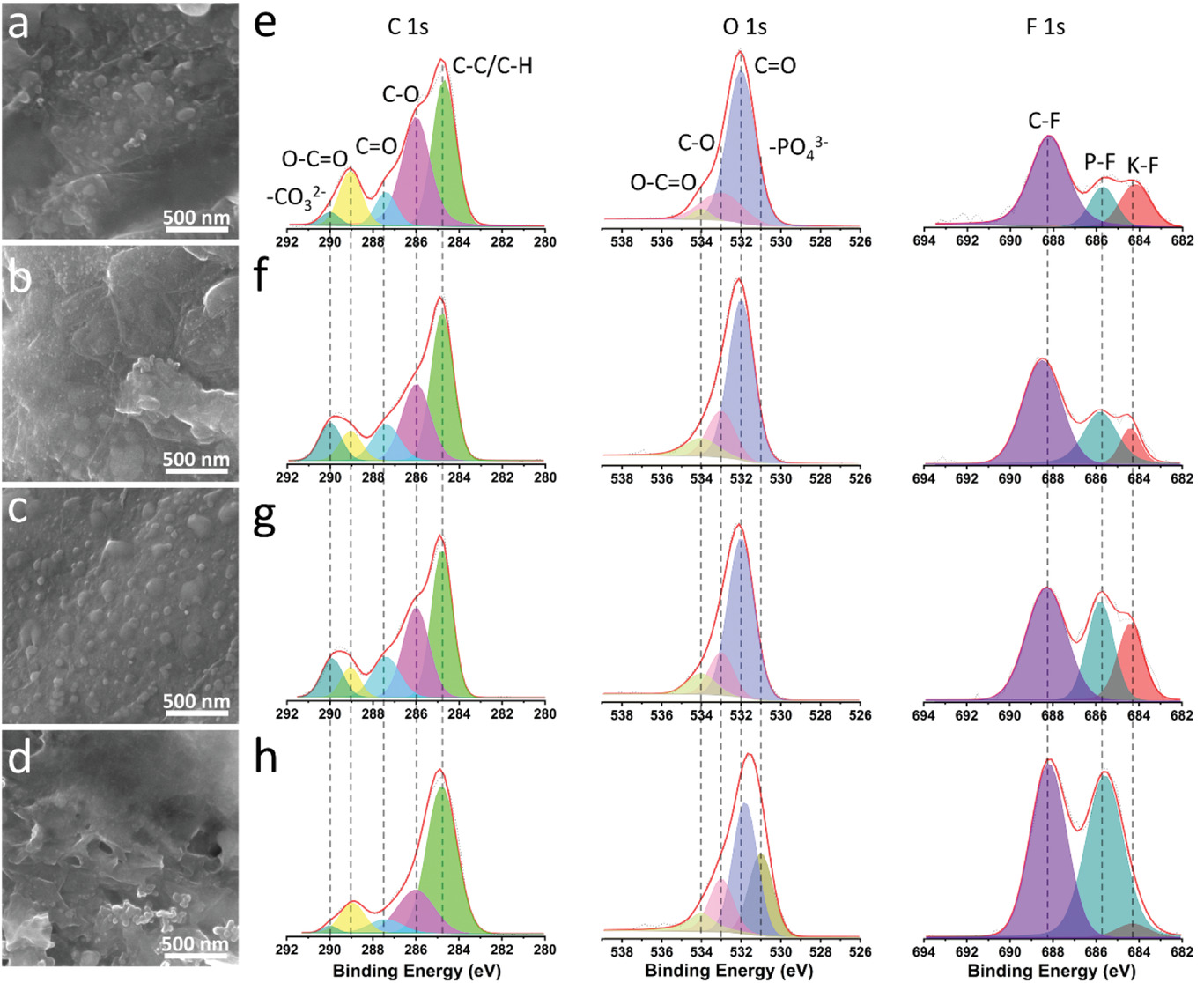
SEM images of the TBPS/NG anode after 20 cycles under the current density of 200 mA g−1 at a) 70 °C, b) 80 °C, c) 90 °C, and d) 100 °C; C 1s, O 1s, and F 1s XPS spectra of the TBPS/NG anode after 20 cycles under the current density of 200 mA g−1 at e) 70 °C, f) 80 °C, g) 90 °C, and h) 100 °C. Image Credit: Qin, K et al., Advanced Science
Conclusions
In conclusion, this study demonstrated a high-temperature, fast-charging, all-organic RPB with a cathode made of PANI and an anode made of TBPS/NG. It was shown that it is feasible to create fast-charging and high-temperature batteries using sustainable and abundant OEMs and potassium resources. For the all-organic RPB, TBPS, an organic anode material, was created and coupled with PANI under harsh circumstances.
The all-organic RPB performed better electrochemically, especially in harsh conditions, when NG and a 2.8 m KPF6-DEGDME electrolyte were used to reduce OEM dissolution, accommodate the significant volume change, improve the conductivity of organic electrodes, and create stable electrode/electrolyte interphases.
The authors mentioned that the development of quick-charging, high-temperature, and environmentally friendly batteries is possible as a result of the obtained results. They believe that the proposed cell architecture shown in this paper holds out a lot of hope for real-world uses of environmentally friendly batteries in harsh environments.
References
Qin, K., Holguin, K., Huang, J., et al. A Fast-Charging and High-Temperature All-Organic Rechargeable Potassium Battery. Advanced Science (2022). https://onlinelibrary.wiley.com/doi/10.1002/advs.202106116.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.