Reviewed by Alex SmithNov 10 2022
A letter titled “A cobalt (II) porphyrin with a tethered imidazole for efficient oxygen reduction and evolution electrocatalysis” was recently published by Rui Cao, Weiqiang Zhang, Shunichi Fukuzumi, Wonwoo Nam, and others in the Journal of Energy Chemistry.
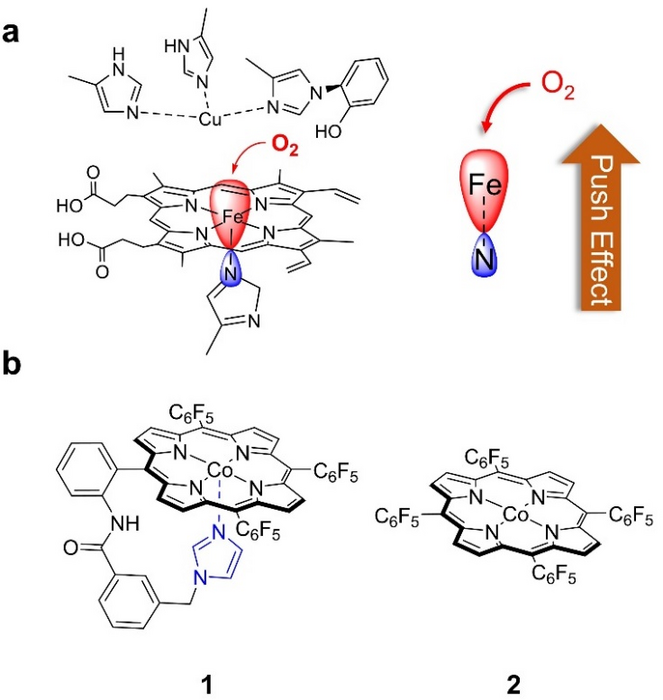
(a) Heme active site in CcOs, showing the trans axial histidine imidazole group and its electronic “push effect” for improved O2 binding and activation. (b) Molecular structures of 1 and 2 studied in this work. Image Credit: Journal of Energy Chemistry
Different fuel cells, metal-air batteries, and water-splitting devices use electrocatalytic oxygen reduction and evolution reactions. Noble metals and their complexes have a low earth abundance and high cost, which restricts their widespread use in industrial applications.
The oxygen reduction reaction (ORR) and the oxygen evolution reaction, however, have recently been linked to a number of first-row transition metal complexes that are useful electrocatalysts (OER). Although it is crucial for their use in rechargeable metal-air batteries, few of these catalysts are effective for both the ORR and OER under the same circumstances.
Co porphyrins have also received a lot of attention as O2 reduction catalysts. Co porphyrins have also been demonstrated to be effective OER electrocatalysts in addition to ORR.
Image Credit: Kaca Skokanova/Shutterstock.com
Despite these accomplishments, more research still needs to be done to create Co porphyrins that are highly active and stable for both the electrocatalytic oxygen reduction and evolution reactions under the same circumstances.
The specific reduction of O2 to water is catalyzed by Fe porphyrins with an axial histidine imidazole group in cytochrome c oxidases (CcOs). By increasing the electron density of the Fe, the axial imidazole group, via an electronic “push effect,” improves O2 binding and activation at the Fe center.
The authors describe a Co porphyrin, 1, with an imidazole group attached at the porphyrin backbone for Co axial binding, which functions as an active bifunctional electrocatalyst for oxygen reduction and evolution reactions.
Additionally, 1 was used as the air electrode’s catalyst in the construction of Zn-air batteries. The resulting Zn-air batteries performed on par with those created using Pt/C and Ir/C materials sold commercially.
This study is significant because it highlights the crucial part that axial ligands play in improving ORR and OER while also because it illustrates the potential uses of molecular catalysts in energy conversion and storage systems based on electrocatalysis.
Journal Reference:
Li, X., et al. (2022) A cobalt (II) porphyrin with a tethered imidazole for efficient oxygen reduction and evolution electrocatalysis. Journal of Energy Chemistry. doi:10.1016/j.jechem.2022.10.010.