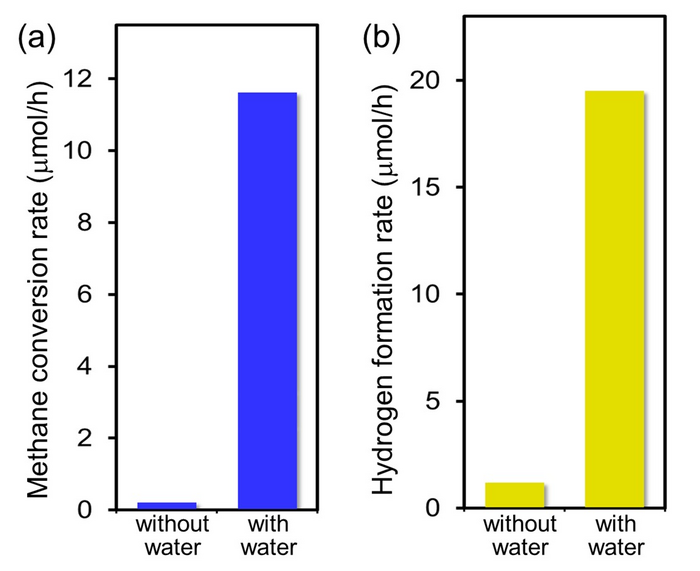
Methane conversion rates and (b) hydrogen formation rates on the Pt/Ga2O3 photocatalysts under ultraviolet irradiation at a methane partial pressure of 70 kPa and water partial pressures of 0 and 2 kPa at a sample temperature of 318 K. The presence of interfacial water significantly enhances photocatalytic activity at ambient temperatures and pressures. Image Credit: NINS/IMS.
Researchers guided by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, recently obtained vital molecular-level insights into the key role of interfacial water in non-thermal C–H activation in photocatalytic methane conversion.
Using real-time mass spectrometry and operando infrared absorption spectroscopy in conjunction with ab initio molecular dynamics simulations, they discovered that methane conversion is rarely stimulated by direct interaction with the trapped hole at the surface Olat site; rather, activation is greatly facilitated by low barrier hydrogen abstraction from methane by the photoactivated interfacial water species.
In contrast to traditional thermocatalytic methane reforming, photocatalytic C–H activation is not the rate-determining step in water-mediated processes. Furthermore, due to the moderate stabilization of •CH3 in the hydrogen-bond network of water, the overall photocatalytic conversion rates are generally increased by more than 30 times at ambient temperatures (~300 K) and pressures (~1 atm).
Unlike thermal catalysis, methane photocatalysis does not require high-pressure methane gas (> 20 atm) in the presence of an adsorbed water layer.
Although water is not explicitly mentioned in the homocoupling reaction equation (2CH4 → C2H6 + H2), the water-assisted repercussions are evident in ethane formation. These findings suggest that interfacial water plays important kinetic roles far beyond the conventional thermodynamic concept of redox potential, in which water oxidation by surface-trapped holes is less thermodynamically preferred than methane oxidation: E°•OH/H2O = 2.73 V and E°•CH3/CH4 = 2.06 V versus the standard hydrogen electrode.
Noticeably, these water-assisted effects are often seen for several representative photocatalysts with different band-gap energies, like TiO2, Ga2O3, and NaTaO3, implying that the incorporation of methane into the photoactivated interfacial hydrogen-bond network is crucial for non-thermal methane activation.
The findings not only aid in the better understanding of non-thermal C–H activation and conversion at the molecular level, but also lay the groundwork for rational interface design of non-thermal catalytic systems for the efficient and sustainable usage of methane under ambient conditions.
Journal Reference
Sato, H., et al. (2023) Critical impacts of interfacial water on C–H activation in photocatalytic methane conversion. Communications Chemistry. doi.org/10.1038/s42004-022-00803-3.