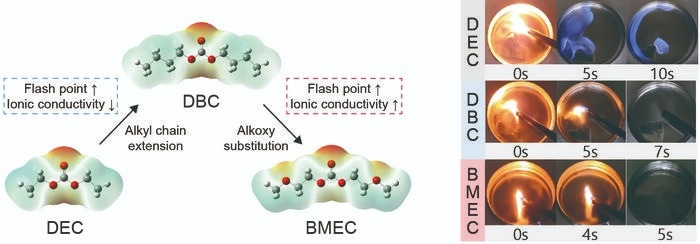
Molecular design strategy for high-flashpoint electrolyte and comparison of room temperature ignition property. Image Credit: Korea Institute of Science and Technology
This announcement was made by The Korea Institute of Science and Technology (KIST, President Seok-Jin Yoon) on behalf of a collaborative research group headed by Dr. Minah Lee of the Energy Storage Research Center, Professor Dong-Hwa Seo of the Korea Advanced Institute of Science and Technology (KAIST), and Drs Yong-Jin Kim and Jayeon Baek of the Korea Institute of Industrial Technology (KITECH).
Since there is an expansion in the use of medium- and large-scale lithium-ion batteries in electric vehicles and energy storage systems (ESS), concerns about fires and explosions are increasing.
Fires in batteries happen when batteries have been subjected to short-circuit as a result of external impacts, aging, or abuse and the thermal runaway phenomenon that comes with a serial exothermic reaction, thereby making it hard to extinguish the fire and look like a high-risk of personal injury.
Especially, the linear organic carbonate that has been utilized in commercial electrolytes for lithium-ion batteries consists of a low flash point and catches fire easily, even at room temperature, which is considered a direct result of ignition.
So far, to decrease the flammability of the electrolyte, intensive fluorination present in the solvent molecules or highly concentrated salts has been adopted extensively. Consequently, the lithium-ion transport in the electrolyte was decreased or inconsistent with commercial electrodes, thereby restricting their commercialization.
By concurrently employing alkyl chain extension and alkoxy substitution to the diethyl carbonate (DEC) molecule, a normal linear organic carbonate utilized in commercial lithium-ion battery electrolytes, the scientists developed a new electrolyte, bis(2-methoxyethyl) carbonate (BMEC), with improved ionic conductivity and flash point by growing the solvation ability and intermolecular interactions.
The BMEC solution consists of a flash point of 121 °C, which is 90 °C greater compared to that of the traditional DEC solution, and thus is not ignitable in the temperature range for conventional battery operation.
BMEC has the potential to dissociate lithium salt stronger compared to its simple alkylated counterpart, dibutyl carbonate (DBC), resolving the issue of slower lithium-ion transport when decreasing flammability by increasing intermolecular interaction.
Consequently, it holds over 92% of the original rate capability of the conventional electrolyte while considerably decreasing the fire hazards.
Besides, the new electrolyte available eased 62% of heat generation and 37% of combustible gas evolution compared to those of the conventional electrolyte. The research group illustrated the stable operation of 1Ah lithium-ion batteries for more than 500 cycles by integrating the new electrolyte with a graphite anode and a high nickel cathode.
Also, they conducted a nail-penetration test on a 70% charged 4Ah-level Li-ion battery and verified the repressed thermal runaway.
The results of this research provide a new direction for designing nonflammable electrolytes, which has been inevitably sacrificed the electrochemical property or economic feasibility.
Dr. Minah Lee, The Korea Institute of Science and Technology
Lee added, “The developed nonflammable electrolyte has cost competitiveness and excellent compatibility with high-energy density electrode materials, so it is expected to be applied to the conventional battery manufacturing infrastructure. Ultimately, it will accelerate the emergence of high-performance batteries with excellent thermal stability.”
Dr. Jayeon Baek of KITECH stated, “The BMEC solution developed in this research can be synthesized by transesterification using low-cost catalysts and easily scaled up. In the future, we will develop the synthesis method using C1 gas (CO or CO2) to enhance its eco-friendliness further.”
Journal Reference
Lee, J., et al. (2023) Molecularly engineered linear organic carbonates as practically viable nonflammable electrolytes for safe Li-ion batteries. Energy & Environmental Science. https://doi.org/10.1039/D3EE00157A.