Image Credit: Tokyo Institute of Technology
The light obtained from the Sun every day, if effectively utilized, can help address the ongoing global energy crisis and also the concerns about climate change. Solar cells, which transform sunlight into electrical energy, are designed with materials that have good photophysical properties, or light absorption.
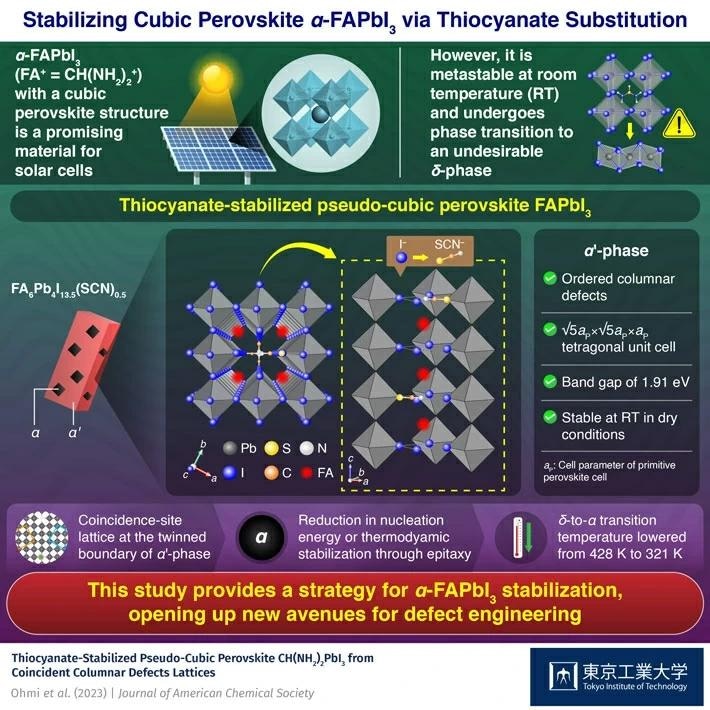
Solar cells made of α-FAPbI3 have a spectacular conversion efficiency of 25.8% and an energy gap of 1.48 eV, both of which are highly desirable for real-world applications. Unfortunately, at room temperature, α-FAPbI3 is metastable and undergoes a phase transition to α-FAPbI3 when stimulated by water or light. Because the energy gap of α-FAPbI3 is much greater than the optimum value for solar cell applications, preserving the -phase is critical for practical purposes.
To address this issue, a group of researchers guided by Associate Professor Takafumi Yamamoto of Tokyo Institute of Technology (Tokyo Tech) recently published a new strategy for stabilizing α-FAPbI3 in the Journal of the American Chemical Society.
By introducing a pseudo-halide anion, thiocyanate (SCN-), the researchers focused on the stabilization mechanism of α-FAPbI3.
Previous studies have shown that partial replacement of surface anions of FAPbI3 from iodide (I–) to SCN– ion stabilizes the α-phase. However, it is still unclear how SCN– ions incorporate themselves within perovskite lattice and increase the interfacial stability.
Takafumi Yamamoto, Associate Professor, Tokyo Institute of Technology
Researchers, for the first time, prepared single crystal and powder samples of the thiocyanate-stabilized pseudo-cubic perovskite. Structural analysis showed that it has a √5-fold superstructure of cubic perovskite with ordered columnar defects, having the α'-phase. The new substance had an energy band gap of 1.91 eV and was discovered to be thermodynamically stable in a dry atmosphere at room temperature.
The presence of the α'-phase in a sample containing the δ-phase favored the δ-to-α phase transformation, lowering the transition temperature by more than 100 K. The scientists noted that the defect-ordered patterns in the α'-phase, that can establish a coincidence-site lattice at the twinned boundary, resulted in the stabilization of the α-phase, either through a reduction in its nucleation energy or thermodynamic stabilization via epitaxy.
These researchers’ discoveries could spur more research into how defect tolerance and vacancy ordering affect the stability of halide perovskites.
This work shows that α-FAPbI3 can be stabilized through pseudo-halide and grain boundary engineering, which might prove beneficial to scientists trying to develop new thermodynamically stable solar cell materials with ideal band gaps and excellent conversion efficiency.
Takafumi Yamamoto, Associate Professor, Tokyo Institute of Technology
Journal Reference:
Ohmi, T., et al. (2023) Thiocyanate-Stabilized Pseudo-cubic Perovskite CH(NH2)2PbI3 from Coincident Columnar Defect Lattices. Journal of American Chemical Society. doi.org/10.1021/jacs.3c05390.