Shaping hard carbon to obtain exceptional large-capacity electrodes for sodium-ion batteries. It is possible to incorporate nanopores in hard carbon by using zinc oxide as a template during its synthesis. These pores enable the material to store many more charge carriers, making it a promising electrode candidate for sodium-ion batteries that can reach an energy density comparable to that of LiFePO4-type lithium-ion batteries. Image Credit: Shinichi Komaba from Tokyo University of Science Japan
Despite their superior performance in various aspects compared to other rechargeable batteries, LIBs come with certain drawbacks. Lithium is a limited resource, and its diminishing availability is expected to drive up prices. Additionally, the extraction of lithium and improper disposal of LIBs present significant environmental challenges, as the liquid electrolytes commonly used in them are toxic and flammable.
The limitations of lithium-ion batteries (LIBs) have spurred global research efforts to explore alternative energy storage technologies. Sodium-ion batteries (NIBs) and potassium-ion batteries (KIBs) have emerged as promising alternatives, offering cost-effective and sustainable solutions. Both NIBs and KIBs are anticipated to become billion-dollar industries by the end of the decade.
Governments worldwide, including the US, Austria, Hong Kong, Germany, and Australia, are actively supporting research and innovation in this field. Notable companies such as Faradion Limited, TIAMAT SAS, and HiNa Battery Technology Co. Ltd. are making substantial investments in these technologies. Electric vehicle battery packs featuring NIBs are expected to be introduced by companies like Contemporary Amperex Technology Co. Limited and build your dreams shortly.
Despite the promise of NIBs and KIBs, the electrode materials’ capacity in these systems still lags behind that of LIBs. Addressing this challenge, a research team led by Professor Shinichi Komaba from Tokyo University Science (TUS), Japan, has been actively working on developing groundbreaking high-capacity electrode materials for NIBs and KIBs.
Their latest study, published in Advanced Energy Materials on November 9th, 2023, introduces a novel synthesis strategy for nanostructured ‘hard carbon’ (HC) electrodes that exhibit unprecedented performance. The co-authors of the study include Mr. Daisuke Igarashi, Ms Yoko Tanaka, and Junior Associate Professor Ryoichi Tatara from TUS, as well as Dr Kei Kubota from the National Institute for Materials Science (NIMS), Japan.
HC is a type of carbon that differs from other forms like graphene or diamond because it is amorphous, and lacks a well-defined crystalline structure. HC is known for its strength and resistance.
In a previous study conducted in 2021, Professor Komaba and his team discovered a method to use magnesium oxide (MgO) as a template during the synthesis of HC electrodes for NIBs. This approach altered the nanostructure of the electrodes, resulting in the creation of nanopores when MgO was removed. This modification significantly increased the electrodes’ capacity to store sodium ions (Na+).
Building on their prior discoveries, the researchers investigated whether compounds composed of zinc (Zn) and calcium (Ca) could serve as effective nano-templates for HC electrodes. In this pursuit, they methodically examined various HC samples created with zinc oxide (ZnO) and calcium carbonate (CaCO3) and conducted a comparative analysis of their performance against those synthesized using magnesium oxide (MgO).
Initial experiments indicated that ZnO held significant promise for serving as the negative electrode material in NIBs. Subsequently, the researchers fine-tuned the concentration of ZnO incorporated into the HC matrix during synthesis.
This optimization resulted in a reversible capacity of 464 mAh g–1 (equivalent to NaC4.8) with a high initial coulombic efficiency of 91.7% and a low average potential of 0.18 V vs. Na+/Na.
By integrating this high-performance electrode material into an actual battery, the research team achieved remarkable results.
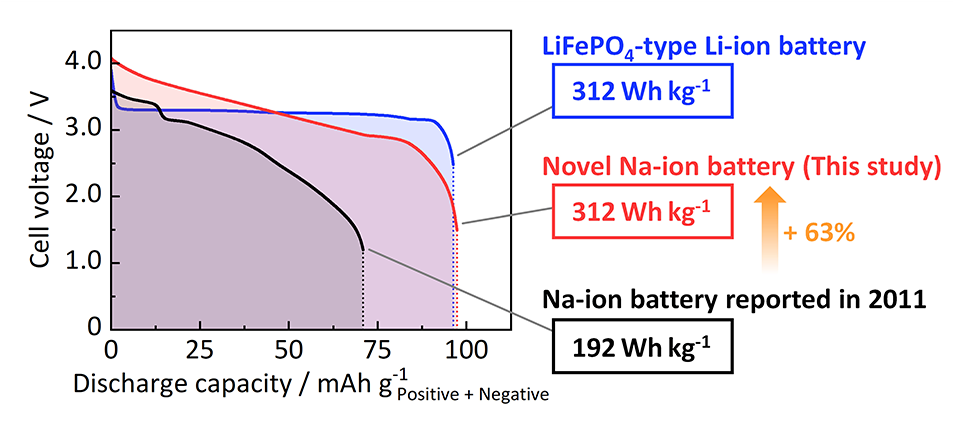
The NIB fabricated using the optimized ZnO-templated HC as the negative electrode exhibited an energy density of 312 Wh kg–1. This value is equivalent to the energy density of certain types of currently commercialized LIBs with LiFePO4 and graphite and is more than 1.6 times the energy density of the first NIBs (192 Wh kg–1), which our laboratory reported back in 2011.
Shinichi Komaba, Author, Department of Applied Chemistry, Tokyo University of Science
Notably, the ZnO-templated HC also exhibited a significant capacity of 381 mAh g–1 when incorporated into a KIB, further showcasing its potential.
In summary, this study's findings suggest that employing inorganic nanoparticles as templates to regulate the pore structure can serve as a valuable guideline in the development of HC electrodes. “Our findings prove that HCs are promising candidates for negative electrodes as an alternative to graphite,” concludes Prof. Komaba.
This development has the potential to render NIBs suitable for practical applications, including the creation of sustainable consumer electronics, electric vehicles, and low-carbon footprint energy storage systems for harnessing energy from solar and wind farms.
Towards Hard Carbon Electrodes for Sodium- and Potassium-Ion Batteries
Video Credit: Tokyo University of Science
Journal Reference
Igarashi, D., et al. (2023). New Template Synthesis of Anomalously Large Capacity Hard Carbon for Na- and K-Ion Batteries. Advanced Energy Materials. doi.org/10.1002/aenm.202302647