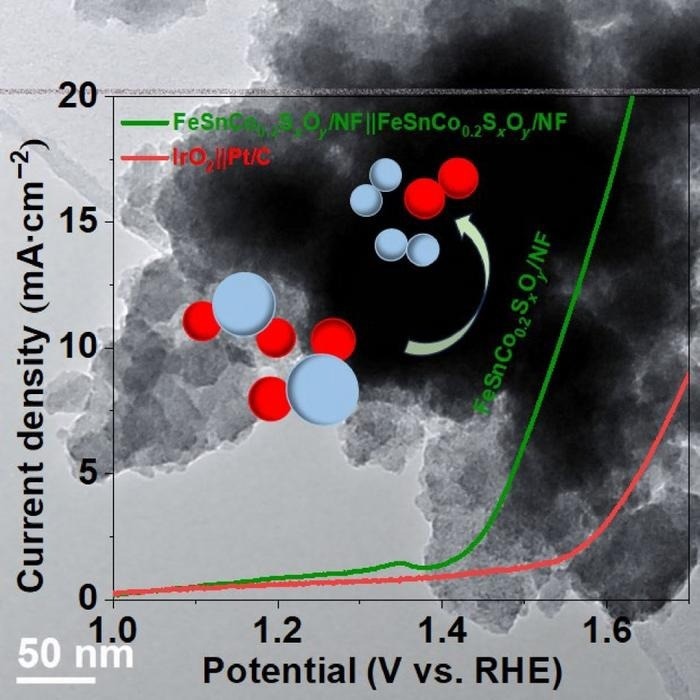
The target of the study, FeSnCo0.2SxOy/NF (green line), requires less voltage to induce oxygen evolution reactions (OER) and hydrogen evolution reactions (HER) than IrO2||Pt/C (red line). Image Credit: Jinqi Guan, Jilin University
By using the simple method of splitting water, hydrogen generation is a quick, safe, and efficient way to generate more energy than gasoline. It is gradually becoming the norm to harvest energy in this way rather than relying primarily (or at all) on carbon-based energy sources.
A technique for creating non-precious metal electrocatalysts for use in economical and ecologically friendly water splitting has been discovered by researchers using transition metal sulfides, such as tin (Sn), cobalt (Co), and iron (Fe), on nickel foam.
Nano Research Energy published the results on November 22nd, 2023.
Certain processes must be stabilized for this procedure to be successful in lowering carbon emissions. FeSnCo0.2SxOy/NF, the study’s main focus, can split water at low voltage by functioning as both an anode and a cathode. This situation concerns two types of reactions: hydrogen evolution reactions (HER) and oxygen evolution reactions (OER). OER uses a chemical reaction to produce O2 from water.
H2 is produced by HER through a two-electron transfer process. The ensuing H2 can be utilized as fuel. To create a bifunctional electrocatalyst, it is best to use both of these reactions. The term “electrocatalyst” refers to catalysts, also known as “reaction-starters,” that work on surfaces that can conduct electricity.
After 55 hours of continuous usage, HER is shown to be stable and needs less overpotential than OER. The discrepancy in the energy required for a particular catalyst to function is known as overpotential.
Regretfully, OER stability does not currently meet requirements. This is caused in part by the additional stage in the electron transfer process as well as the often harsh electrolytes in which they operate. OER’s activity does diminish with increasing cobalt content, even though it remains steady for about 70 hours of continuous use.
It is pivotal to improve the OER stability of transition metal sulfides so that they can be used as bifunctional HER and OER catalysts for reversible hydrogen fuel cell.
Jingqi Guan, Study Author and Researcher, Jilin University
In comparison to HER, OER has a higher overpotential. OER could seem more “tough” if more energy input is required to activate the catalyst. On nickel foam, iron, tin, and cobalt together offer some improvements in HER and OER activity as well as bifunctional stability.
The distribution of electrons over the electrolyte surface can be altered by the mix of these metals and the heterostructural interfaces that are created. In this context, “heterostructural” describes a semiconductor that can change its chemical makeup depending on where two compounds are positioned. Here, the combination is sulfide/oxyhydroxide.
A uniform distribution of electrons facilitates the transmission of electrons by speeding up the rate at which charges are transferred throughout the entire structure. Owing to the nature of this semiconductor, general activity and function would inevitably improve with increased stability.
All things considered, these transition metals work in concert with one another, particularly during HER. Because of this effect, they are the best candidates for the researchers’ primary aim, which is to decrease the use of carbon-based energy sources.
Even if the outcomes were quite encouraging, process improvement can always be pursued in the future. The amount of energy required to catalyze the reaction can be decreased by using a catalyst that minimizes overpotentials. The long-term viability of the heterostructural interfaces also depends on making sure the electrocatalysts produced are strong enough to be employed commercially and can tolerate extended periods of continuous use without experiencing any negative side effects.
Contributors to this study included Changmin Hou of the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry at Jilin University, Hui Qi of the Second Hospital of Jilin University, Siyu Chen, Ting Zhang, Jingyi Han, Shihui Jiao, and Jingqi Guan of the Institute of Physical Chemistry at Jilin University.
The study was supported by the Natural Science Foundation of Jilin Province and the National Natural Science Foundation of China.
Journal Reference
Chen, S., et. al. (2023) Interface engineering of Fe-Sn-Co sulfide/oxyhydroxide heterostructural electrocatalyst for synergistic water splitting. Nano Research Energy. doi:10.26599/NRE.2023.9120106