One of the main obstacles to effective renewable energy storage and large carbon emission reductions is battery capacity. In a lithium-ion battery (LIB), Tin (Sn) and Sn-mixture alloys could be used as a battery anode that releases electrons, potentially storing more energy at a higher density than more typical carbon-based anodes.
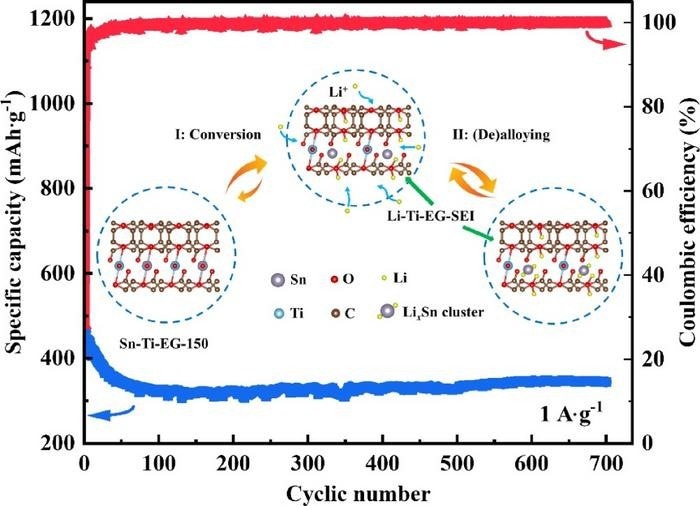 The Sn-Ti-EG anode maintained a capacity of 345 mAh g-1 (blue line) at a current density of 1.0 A g-1 after 700 charge-discharge cycles. The metal-organic framework (MOF) of the Sn-Ti-EG anode mitigates the typical stability issues of Sn and Sn-alloy anodes that occur due to expansion during the charge-discharge cycle. Image Credit: Energy Materials and Devices, Tsinghua University Press
The Sn-Ti-EG anode maintained a capacity of 345 mAh g-1 (blue line) at a current density of 1.0 A g-1 after 700 charge-discharge cycles. The metal-organic framework (MOF) of the Sn-Ti-EG anode mitigates the typical stability issues of Sn and Sn-alloy anodes that occur due to expansion during the charge-discharge cycle. Image Credit: Energy Materials and Devices, Tsinghua University Press
When a Sn-Ti bimetal element was combined with cheap ethylene glycol (Sn-Ti-EG), many of the difficulties associated with employing Sn as an anode material were reduced, and an affordable LIB with superior storage and performance qualities was created.
While Sn and Sn alloy mixtures of Sn and another metal may perform better than alternative anode materials, their low stability is caused by the expansion of the metal during charging and discharging.
Developing a metal-organic framework (MOF) that offers strong stability while charging and recharging and maintains quick electron transfer (energy flow) is one method to get around this restriction.
A Sn-Ti-EG bimetal organic compound MOF was recently developed by materials scientists, and it showed excellent electricity conduction, energy capacity, and stability across a number of charging and discharging cycles. On November 20th, 2023, the researchers published their findings in the journal Energy Materials and Devices.
Significant efforts have been directed toward developing high-capacity cathode and anode materials for high energy-density LIBs. Because the capacities of well-known cathode materials, for example, LiFePO4, Ni-rich layered oxides, and LiMn2O4, have reached their theoretical limits, more attention is being focused on finding anode materials that have high energy densities as a substitute for the commonly used graphite anodes that have a relatively low theoretical capacity and tap density.
Zhen-Dong Huang, Senior Author and Professor, State Key Laboratory for Organic Electronics and Information Displays
Zhen-Dong Huang is also associated with the Jiangsu Key Laboratory for Biosensors at Nanjing University of Posts and Telecommunications in Nanjing, China.
The amount of electric charge (milliampere hours, or mAh) that a material can deliver per gram (g-1) is known as its capacity, and anodes composed of graphite, a crystalline form of carbon, have a theoretical capacity of 372 mAh g-1.
On the other hand, compared to graphite anodes, the theoretical capacities of Sn, Bismuth (Bi), and Antimony (Sb) metals are higher. For instance, sn anodes have a theoretical capacity of 994 mAh g-1, but their growth causes stability problems.
Huang said, “To resolve the stability issues associated with Sn anodes, a myriad of strategies have been explored, including minimizing the particle size, introducing inert metals, and assembling with carbon materials. Moreover, rationally designed structures, such as hollow, layered, and core–shell structures, play an important role in alleviating volume expansion.”
Further, Huang continued, “Although these strategies helped the cyclic stability to a certain degree, the… energy densities of the nanostructured Sn-based anodes are normally low. In contrast, metal-organic frameworks have an intrinsically porous structure that not only provides a large number of active sites but also enables rapid electrolyte penetration and electron/ion transfer.”
To produce a more stable anode material with excellent electrochemical performance, the research team developed a special MOF composed of Sn, Ti, and EG. For instance, EG completed the battery circuit by acting as an organic bridge between the positively charged Sn2+ and Ti4+ ions.
Ti additionally added to the material's enhanced stability and structure. Sn improved the electrochemical performance of the anode material with its larger theoretical capacity. The researchers ultimately developed a new, low-cost LIB anode material that, even after 700 cycles, demonstrated the stability of the anode material by maintaining a high specific capacity of 345 mAh g-1 at a current density of 1000 mA g−1.
After 700 cycles, images captured with a scanning electron microscope verified that the anode material was free of fractures. The strong interaction between the Sn and carbon-oxygen species was found to be the cause of the electrode's high specific capacity and excellent cyclic stability, according to further analysis of the Sn-Ti-EG anode material.
This information could aid future researchers in creating anode materials with comparable properties. The current development in anode-specific capacity, according to the study team, is a precursor to other LIB materials that can enhance battery storage capacity and be mass-produced profitably.
Yuqing Cai, Haoran Li, Qianzi Sun, Xiang Wang, and Ziquan Li from the State Key Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors at the Institute of Advanced Materials in the Jiangsu National Synergetic Innovation Center for Advanced Materials at the Nanjing University of Posts and Telecommunications in Nanjing, China contributed to the research.
Other contributors included Haigang Liu from the Shanghai Synchrotron Radiation Facility in the Shanghai Advanced Research Institute at the Chinese Academy of Sciences in Shanghai, China; and Jang-Kyo Kim from the Department of Mechanical Engineering at Khalifa University in Abu Dhabi, United Arab Emirates.
The study was funded by the National Natural Science Foundation of China, the Project of State Key Laboratory of Organic Electronics and Information Displays, Nanjing University of Posts and Telecommunication, the Postgraduate Research & Practice Innovation Program of Jiangsu Province, Khalifa University financial support, and the Shanghai Synchrotron Radiation Facility.
Journal Reference:
Cai, Y., et.al., (2023). Strong coordination interaction in amorphous Sn-Ti-ethylene glycol compound for stable Li-ion storage.Energy Materials and Devices. doi.org/10.26599/emd.2023.9370013.