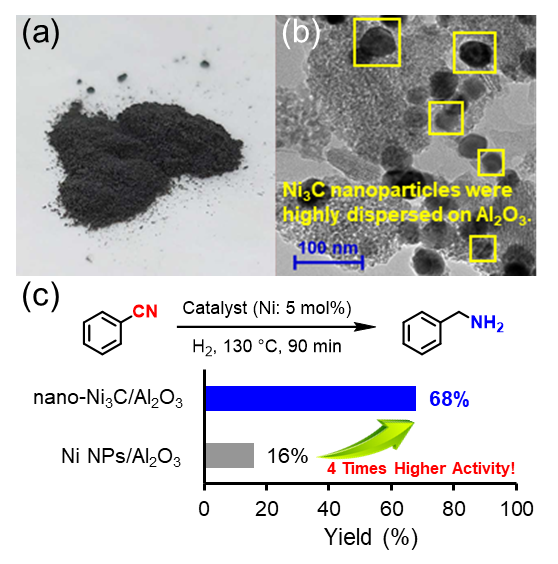
(a) The photo of nano-Ni3C/Al2O3 catalyst. (b) Transmission electron microscope image of nano-Ni3C/Al2O3. (c) Hydrogenation of nitrile to benzylamine using nano-Ni3C or Ni nanoparticle catalyst, and the substrate scope for the nitrile hydrogenation catalyzed by nano-Ni3C/Al2O3. Image Credit: 2024 Yamaguchi et al., Chemistry – A European Journal
This discovery could spur further attempts to reduce costs in the chemical industry.
Rare and costly metals are frequently used by the chemical industry to make prescription drugs and other necessary chemicals. It would be more cost-effective, less risky of supply chain disruptions, and environmentally sustainable to replace these metals whenever possible with more affordable, more plentiful alternatives.
Researchers from Osaka University and its partners have now addressed this need in the work on an industrially useful chemical transformation, which was recently published in Chemistry - A European Journal. Researchers trying to cut back on the amount of costly metals used in as many chemical reactions as possible may find inspiration in the straightforward, gentle reaction conditions described here.
Particularly adaptable materials are those referred to as noble metals. For instance, palladium is the preferred metal for catalyzing a chemical reaction that is frequently involved in the manufacturing of nylon and plastics: turning nitriles into primary amines. These metals are pricey and uncommon, though.
Catalyst alternatives based on common metals, like nickel, might be less expensive. Unfortunately, for the aforementioned chemical transformation, many inexpensive metals require difficult experimental settings, like high pressures and temperatures. The research team’s objective was to ascertain whether nickel carbide has the same restrictions and, if not, to assess the range of chemical transformations that can occur with this catalyst.
In our work, we thoroughly study the reaction chemistry that underlies a novel nickel carbide nanoparticle heterogeneous catalyst for selective hydrogenation of nitriles into primary amines. The substrate scope is broad – many types of heteroaromatic and aliphatic nitriles can undergo this transformation.
Sho Yamaguchi, Lead Author, Department of Materials Engineering Science, Graduate School of Engineering Science, Osaka University
The catalyst developed by the researchers has various benefits. One is that the catalyst demonstrated four times the activity of basic nickel nanoparticles, even under the mild reaction conditions that were required, which included one atmosphere of hydrogen pressure and a relatively low temperature of about 150 ºC. Two, the catalyst could be reused three times or more. Three, up to 99% of the reactions had high yields.
We’re excited because our research will help minimize the use of expensive metals for and simplifies the experimental setup of a common class of chemical syntheses. Furthermore, our theoretical calculations provide insights that will help us optimize the catalyst for additional applications.
Tomoo Mizugaki, Senior Author, Department of Materials Engineering Science, Graduate School of Engineering Science, Osaka University
This work is a significant advancement toward making a class of chemical reactions more sustainable, which is needed to synthesize many other common products, including pharmaceuticals. Applications to additional chemical transformations should be feasible because the nickel catalyst is much less expensive than a noble metal, and the necessary experimental procedures are straightforward.
Journal Reference
Yamaguchi, S., et al. (2023). Nickel Carbide Nanoparticle Catalyst for Selective Hydrogenation of Nitriles to Primary Amines. Chemistry - A European Journal. doi.org/10.1002/chem.202303573