Reviewed by Lexie CornerMay 19 2025
Researchers from Yanshan University, the Chinese Academy of Sciences, and the Institute of Process Engineering (IPE) have developed a novel gold-catalyzed method for engineering atomically rough surfaces (ARSs) on gold-based binary alloys. This approach significantly enhances electrocatalytic performance in the ethanol oxidation reaction (EOR), a key process in direct alcohol fuel cells.
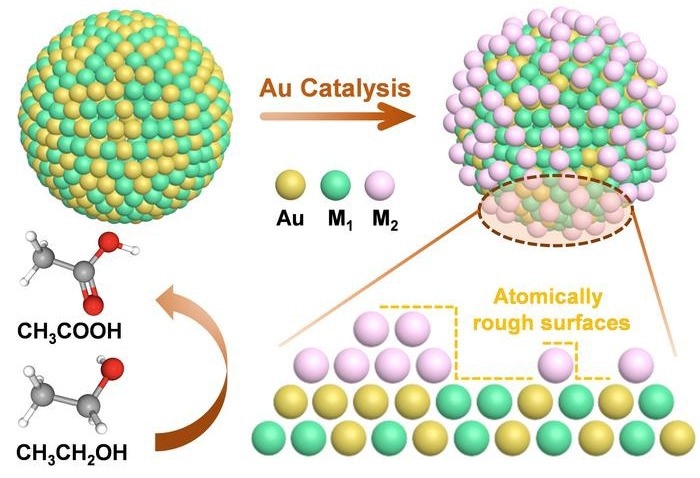 Schematic illustration showing the construction of atomically rough surfaces (ARSs) by Au-catalyzed reduction of metal ions. Image Credit: By YANG’s group
Schematic illustration showing the construction of atomically rough surfaces (ARSs) by Au-catalyzed reduction of metal ions. Image Credit: By YANG’s group
This approach may support the development of more efficient and stable fuel cell technologies.
The EOR is a complex, multi-step process that is highly sensitive to the atomic structure of electrocatalyst surfaces. Currently, the most effective EOR catalysts are based on platinum (Pt) or palladium (Pd) nanomaterials. However, intermediate species can strongly adsorb onto these surfaces, blocking active sites and reducing catalytic efficiency. Surface engineering, alloying, and shape or size control are common strategies to address these issues.
In this study, researchers synthesized gold-based bimetallic nanoalloys (AuM1, where M1 = Pd or Ag) and then deposited a second metal (M2 = Pd, Pt, or Cu) onto the surface. This process forms ARSs, facilitated by the ligand effect of neighboring Au atoms.
The nanoparticles were characterized using transmission electron microscopy (TEM), high-resolution TEM (HRTEM), and X-ray photoelectron spectroscopy (XPS). The data confirmed the formation of homogeneous alloys and the surface deposition of secondary metal atoms, resulting in ARSs.
Electrochemical tests showed that AuPd-Pt nanoparticles had the highest EOR performance, with a specific activity of 14.9 mA cm-2 and mass activity of 28.5 A mg–1. These results exceed those of AuPd alloys, commercial Pd/C, and other recent Pd-based catalysts.
In situ Fourier transform infrared (FTIR) spectroscopy and density functional theory (DFT) calculations indicated that the Pd-Pt ARSs favor incomplete oxidation to acetate rather than full oxidation to carbon dioxide.
The improved performance of AuPd-Pt nanoparticles can be due to numerous variables. The ARSs include an abundance of low-coordinated atoms, which serve as highly active sites with low energy barriers for reactant activation.
The improved performance is attributed to several factors: a high density of low-coordinated atoms on ARSs, which offer active sites with lower energy barriers; electronic structure modification via Au's ligand effect, which weakens intermediate binding; and faster kinetics in the partial oxidation pathway, which is less affected by mass transport and poisoning effects than full oxidation routes.
This research not only demonstrates a new method for constructing ARSs but also provides insights into the design of nanostructures for electrocatalysis.
Jun Yang, Study Corresponding Author and Professor, Institute of Process Engineering (IPE), Chinese Academy of Sciences
The findings have potential implications for improving the efficiency of fuel cells and other electrochemical energy conversion systems.
However, the study has limitations. The primary focus was on demonstrating the fabrication of atomically rough surfaces (ARSs) and evaluating their influence on the ethanol oxidation reaction (EOR) pathway. A complete analysis of the reaction products was not conducted.
Future work will aim to assess Faradaic efficiency more comprehensively using advanced characterization techniques, including ion chromatography (IC), high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR) spectroscopy, and gas chromatography (GC).
This gold-catalyzed approach offers a promising direction for improving catalyst performance in electrochemical applications and warrants further investigation in broader reaction contexts.
Journal Reference:
Zeng, Q., et al. (2025). Gold-catalyzed construction of atomically rough surfaces towards high-efficiency ethanol electrooxidation. Science Bulletin. doi.org/10.1016/j.scib.2025.04.051