Reviewed by Lexie CornerMay 23 2025
A research team has developed a comprehensive theoretical model to more accurately predict the performance of single-atom catalysts (SACs) in the electrochemical reduction of carbon dioxide (CO2RR). The model uniquely incorporates the effects of both pH and the interfacial electric field - two critical factors often overlooked or simplified in conventional catalyst studies.
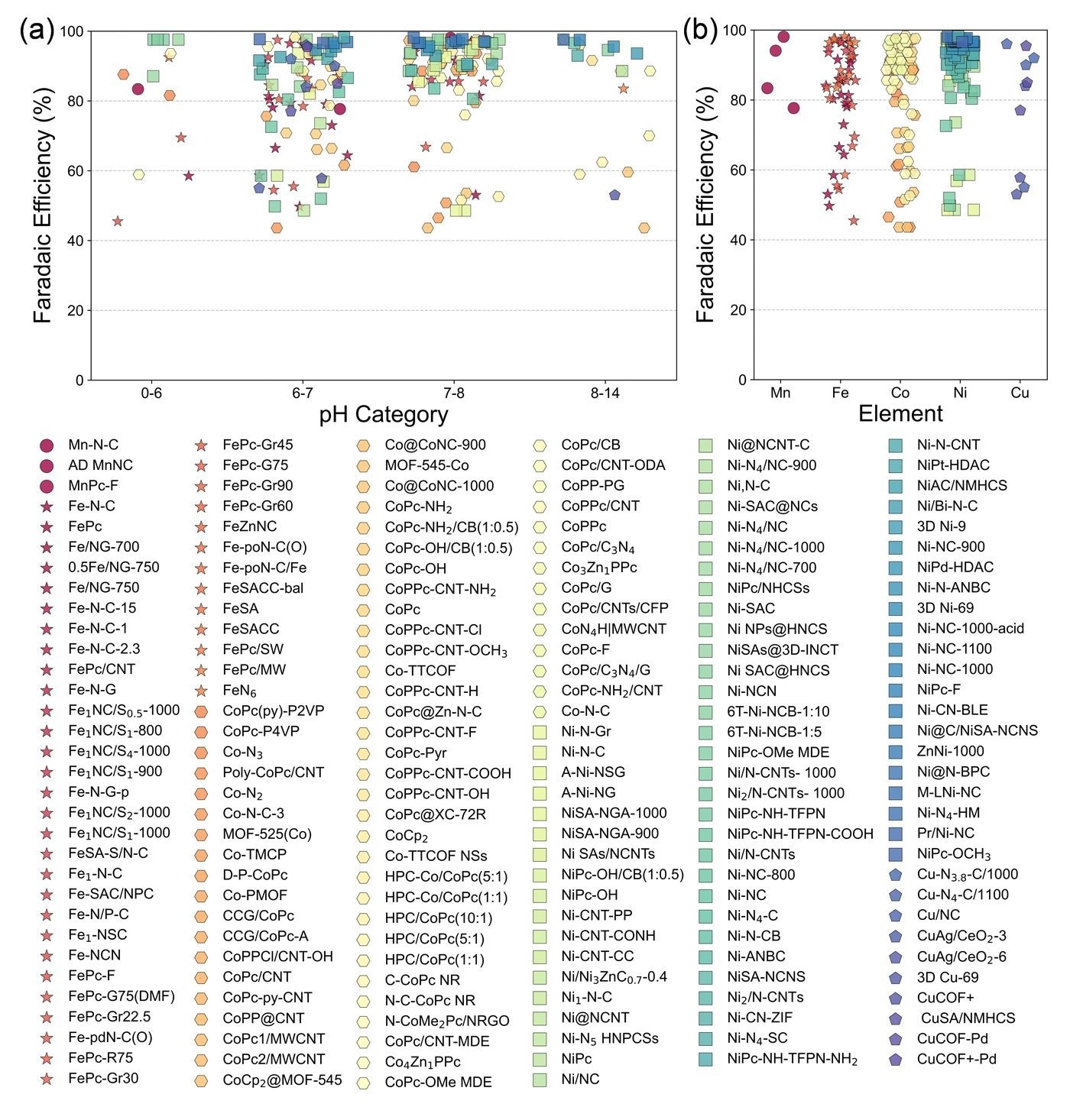 Activity analysis of 230 M-N-C catalysts for CO production through the CO2RR, compiled via large-scale data mining. Image Credit: Hao Li et al.
Activity analysis of 230 M-N-C catalysts for CO production through the CO2RR, compiled via large-scale data mining. Image Credit: Hao Li et al.
Electrochemical CO2RR is being explored as a way to convert carbon dioxide into useful chemicals under mild conditions. One key product is carbon monoxide (CO), which is used in syngas and chemical manufacturing. However, catalyst performance in CO2RR is strongly affected by the surrounding environment, especially the pH of the electrolyte.
To better understand and predict catalyst behavior, a research team developed a microkinetic model based on the reversible hydrogen electrode (RHE) scale. The model includes the effects of pH and interfacial electric fields - two factors that are often overlooked in catalyst design. They combined the model with spin-polarized density functional theory (DFT-D3) calculations and data screening to evaluate 101 single-atom catalyst (SAC) configurations with different d-block transition metals.
The study looked at SACs in various coordination environments, such as graphene with pyrrole and pyridine groups, covalent organic frameworks (COFs), porphyrins, and phthalocyanines. They found a consistent linear relationship between how strongly COOH and CO intermediates bind to the surface, suggesting a predictable trend across different catalysts.
One key insight was that dipole–field interactions help explain why certain catalysts perform differently under varying pH conditions. These effects influence how reaction intermediates bind to the surface.
The model’s predictions closely matched available experimental data on turnover frequency. Based on this, the researchers identified 12 SACs that show good selectivity for CO production across a wide pH range. Most of these promising candidates used iron (Fe), copper (Cu), or nickel (Ni) as the central metal atom.
This work represents a step toward more predictive catalyst screening by bridging theory with realistic electrochemical conditions. By integrating pH and field effects into our model, we can more accurately guide the design of catalysts suited for carbon-neutral energy technologies.
Hao Li, Professor and Study Lead Author, Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
The computational models used in this study are available on the Digital Catalysis Platform - an open-access resource developed by Professor Li's group to support collaboration and data sharing in the catalysis field.
The researchers plan to improve the model by increasing its accuracy and making it more computationally efficient. They also aim to incorporate machine learning to speed up catalyst design and better understand pH-sensitive electrochemical reactions.
Journal Reference:
Chu, Y., et al. (2025) Data-driven discovery of single-atom catalysts for CO2 reduction considering the pH-dependency at the reversible hydrogen electrode scale Editor’s Pick. The Journal of Chemical Physics. doi.org/10.1063/5.0267969