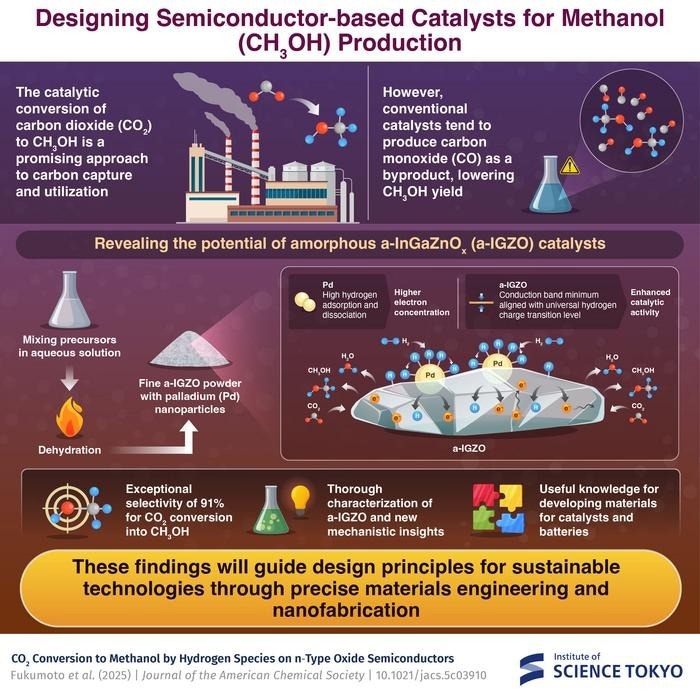
Japanese researchers revealed that a novel palladium-loaded amorphous InGaZnOx (a-IGZO) catalyst converted carbon dioxide to methanol with over 91% selectivity. The study was published in the Journal of the American Chemical Society.
 The study sheds light on a new strategy to design semiconductor-based catalysts for challenging reactions, including carbon capture via carbon dioxide conversion to methanol. Image Credit: Institute of Science Tokyo
The study sheds light on a new strategy to design semiconductor-based catalysts for challenging reactions, including carbon capture via carbon dioxide conversion to methanol. Image Credit: Institute of Science Tokyo
This technology, in contrast to conventional catalysts, uses semiconductors’ electrical characteristics to produce every species required for the conversion reaction. The study presents new electronic structure engineering-based design concepts for sustainable catalysis.
The global effort for carbon neutrality depends on the capacity to absorb CO2 and convert it into lucrative resources. One of the most promising approaches is to convert CO2 into methanol (CH3OH), a vital building ingredient in the chemical industry and a possible clean energy carrier in a hydrogen-based economy.
While this strategy presents a compelling opportunity to reduce greenhouse gas emissions while providing wealth, technological obstacles remain.
Conventional catalysts for CO2-to-CH3OH conversion, such as those based on copper-zinc oxide complexes, have low selectivity. They frequently create undesired carbon monoxide (CO) as a byproduct, reducing CH3OH output and undermining efficiency and environmental advantages. This has motivated researchers to look into other catalyst design methodologies that exploit semiconductor materials' intrinsic electronic properties.
In a recent study, a research team led by Professor Hideo Hosono from the MDX Research Center for Element Strategy at the Institute of Science Tokyo (Science Tokyo), Japan, proposed a revolutionary technique to overcome current restrictions.
The findings show that n-type oxide semiconductors could be designed as extremely effective catalysts for CO2-to-CH3OH conversion. Professor Masaaki Kitano and Assistant Professor Masatake Tsuji, both from Science Tokyo, co-authored this study, which was carried out in partnership with Mitsubishi Chemical Corporation.
The researchers concentrated on amorphous indium-based oxides, namely a-InGaZnOx (a-IGZO), which is commonly employed as a semiconductor to drive pixels in display technology. They created fine powders of these oxides to enhance surface area, an important component in catalytic activity. The scientists next assessed the produced materials' catalytic efficacy, separately and in combination with palladium (Pd) nanoparticles.
The crucial discovery was understanding how the electrical structure of these semiconductor catalysts promotes the required conversion process. Unlike typical catalysts, which rely heavily on surface chemistry, the a-IGZO system has distinct electronic properties.
The conduction band minimum corresponds to the 'universal hydrogen charge transition level (UHCTL), an energy level in semiconductors where H+ and H− ions are equally stable. UHCTL is around 4.5 eV from the vacuum level.
This alignment enables the catalyst to produce both positively and negatively charged hydrogen species, which are required for the multi-step process of turning CO2 into CH3OH.
The Pd nanoparticles act as hydrogen providers, converting hydrogen molecules into atomic hydrogen (H0) and delivering them to the semiconductor surface. High carrier concentration in oxide semiconductors allows H0 to tunnel over the Schottky barrier at the Pd/semiconductor contact.
Thanks to these processes, the Pd-loaded a-IGZO catalyst obtained over 91% selectivity for CH3OH generation, which is a significant improvement over traditional systems.
Our work shows that realization of bipolar state (H+ and H−) of hydron is a key to efficient and highly selective methanol synthesis from CO2, and the design principle for the catalyst is to choose n-type oxide semiconductors with conduction band minimum close to UHCTL, and high carrier concentration.
Hideo Hosono, Professor, MDX Research Center for Element Strategy, Institute of Science Tokyo
Overall, the suggested semiconductor-based method can potentially represent a paradigm change in catalyst design, moving away from traditional surface chemistry-focused tactics and toward new ones based on electronic structure.
“Our findings not only demonstrate the effectiveness of utilizing electrons, holes, hydrogen species, and their dynamics within semiconductors for CO2 hydrogenation, but also suggest new design guidelines for chemical devices such as catalysts and batteries,” concludes Hosono.
These discoveries should spur the development of more effective carbon capture and utilization systems.
Journal Reference:
Fukumoto, K., et al. (2025) CO2 Conversion to Methanol by Hydrogen Species on n-Type Oxide Semiconductors. Journal of the American Chemical Society. doi.org/10.1021/jacs.5c03910.