|
The special nature of carbon combines with the molecular perfection of buckytubes (single-wall carbon nanotubes) to endow them with exceptionally high material properties such as electrical and thermal conductivity, strength, stiffness, and toughness. No other element in the periodic table bonds to itself in an extended network with the strength of the carbon-carbon bond. The delocalised pi-electron donated by each atom is free to move about the entire structure, rather than stay home with its donor atom, giving rise to the first molecule with metallic-type electrical conductivity. The high-frequency carbon-carbon bond vibrations provide an intrinsic thermal conductivity higher than even diamond.
In most materials, however, the actual observed material properties - strength, electrical conductivity, etc. - are degraded very substantially by the occurrence of defects in their structure. For example, high strength steel typically fails at about 1% of its theoretical breaking strength. Buckytubes, however, achieve values very close to their theoretical limits because of their perfection of structure - their molecular perfection. This aspect is part of the unique story of buckytubes.
Buckytubes are an example of true nanotechnology: only a nanometer in diameter, but molecules that can be manipulated chemically and physically. They open incredible applications in materials, electronics, chemical processing and energy management.
|
|
Buckytubes are single-wall carbon nanotubes, in which a single layer of graphite - graphene - is rolled up into a seamless tube. Graphene consists of a hexagonal structure like chicken wire. If you imagine rolling up graphene or chicken wire into a seamless tube, this can be accomplished in various ways. For example, carbon-carbon bonds (the wires in chicken wire) can be parallel or perpendicular to the tube axis, resulting in a tube where the hexagons circle the tube like a belt, but are oriented differently. Alternatively, the carbon-carbon bonds need not be either parallel or perpendicular, in which case the hexagons will spiral around the tube with a pitch depending on how the tube is wrapped. Figure 1 illustrates these points:
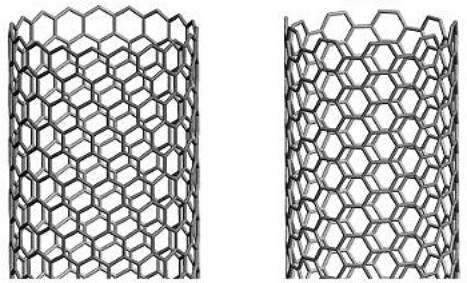
Figure 1. Buckytube structures
Naming Convention
There is a straightforward labelling convention to distinguish differently wrapped tubes from one another, illustrated by Figure 2. The mapping specifies the number of unit vectors required to connect two atoms in the planar hexagonal lattice to form a seamless tube. These numbers specify a "vector" for the mapping, commonly expressed as (m,n), where m and n are integers. These numbers constitute a unique "name" for a tube. Any tube "named" (n,0) has carbon-carbon bonds that are parallel to the tube axis, and form, at an open end, a "zig-zag" pattern; these tubes are referred to as "zig-zag" tubes. Tubes named (n,n), where the two integers are equal, have carbon-carbon bonds that are perpendicular to the tube axis, and are often called "armchair" tubes. These two basic types are achiral, meaning they do not have a distinct mirror-image, like left and right hands. All the other tubes, named (m,n), where m does not equal n, and neither is 0, are chiral, and have left-and right-handed variants.
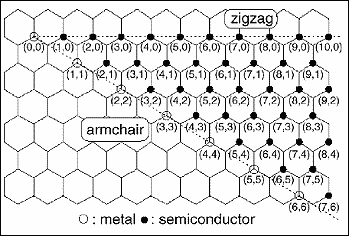
Figure 2. Labelling convention for buckytubes
Properties of Different Tube Types
In most respects, the properties of tubes of different types are essentially the same. The exception to this is in their electrical conductivity, where these subtle structural differences can have profound effects. For example, all armchair tubes - i.e., where m=n - have truly metallic electrical conductivity.
They transport electrons along the tube axis just as metals do, without a single atom of metal in their structure! This behaviour in a molecule is unprecedented. In contrast, the other tubes are intrinsically semiconducting, either with a very small band gap of a few meV, or with moderate band gaps on the order of 1 eV. The rule here is that those tubes where (n-m) is a multiple of 3 are the small-gap type, while the others have medium gaps.
Since tubes with different (n,m) are molecularly distinct, there exists the possibility of chemically separating different types, and even of growing only selected tube types, although currently, all production processes produce a random mixture.
Ropes
Another structural aspect of tubes is their self-organization into "ropes," which consist in many (typically, 10-100) tubes running together along their length in van der Waals contact with one another. Ropes are far longer than any individual tube in them: whereas tubes are typically about 100-1000 nm in length (and about 1 nm in diameter) ropes are essentially endless, branching off from one another, then joining others. These ropes are useful in providing very long conductive pathways well below what would normally be the loading required to reach a "percolation threshold" for conductivity.
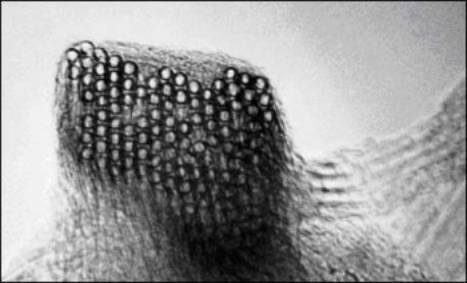
Figure 3. Buckytube ropes
|