
In this interview, AZoM speaks to Pierre Burgos, AFM-Raman product specialist at HORIBA Scientific, about the applications, advantages, and limitations of TERS.
Why is TERS analysis of lipid bilayers interesting for nanotoxicological studies?
The membrane structure of every cell is made of lipid bilayers. Animal, human, and bacteria membranes are all made of a lipid bilayer, which acts as the border between the inside and outside of the cell. It is integral to protecting the cell, and yet must allow chemicals and nutrients to cross the membrane; furthermore, potentially harmful organisms such as a virus can enter cells through the membrane. Understanding the chemical structure of cell membranes is key to understanding disease, toxicology, nutrition, and general cell health.
Laboratory-prepared phospholipid bilayers are a good chemical analog to cell membranes – they possess the same overall chemical structure including the fundamental bilayer structure, but can be prepared and chemically modified through standard laboratory techniques.
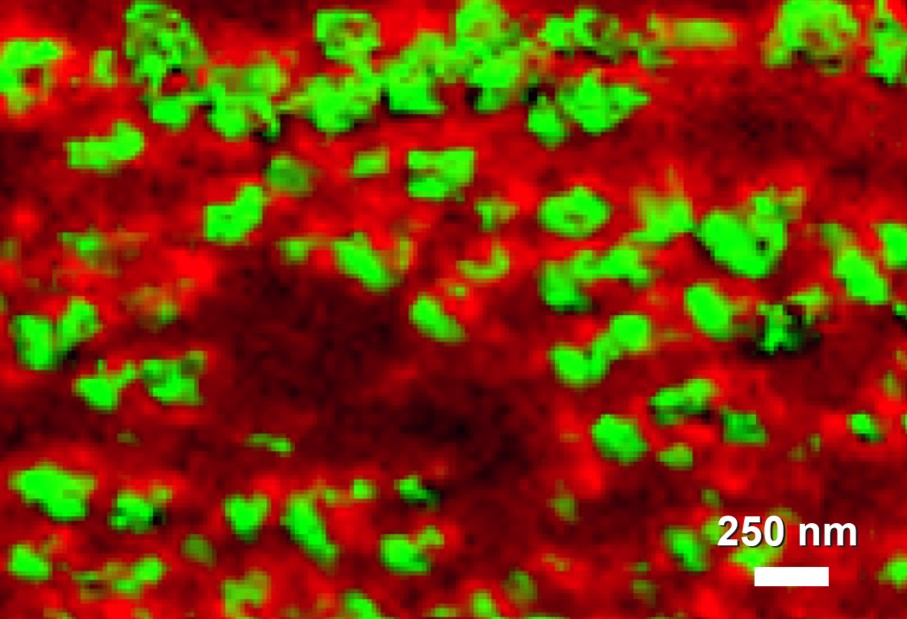
Image showing 20-50 nm domains of phospholipid bilayers showing molecular orientation differences visualized with tip-enhanced Raman spectroscopy (TERS)
There is growing interest in the fate of nanomaterials within the environment, as the uptake of these within commercial devices and processes increases. Nanomaterials are of a size that can closely interact with cell membranes, and potentially penetrate cells…as a result, the toxicological impact of such materials could be significant, but there has been little work on such topics.
As part of the Horizon 2020 ACEnano consortium, HORIBA has been investigating how TERS can help to understand this important nanotoxicology question. The ACEnano project aims to introduce confidence, adaptability, and clarity into nanomaterial risk assessment though physicochemical nanomaterial characterization.
With HORIBA’s tip-enhanced Raman spectroscopy (TERS) systems it is possible to probe both the lipid bilayers and individual nanoparticles, benefiting from the non-destructive chemically rich characterization of Raman whilst working with a sub-20 nanometer spatial resolution. TERS can offer an understanding of both the bilayer structure (and thus shed light on cell membranes themselves) and the impact of nanomaterials…do they interact, do they penetrate? Such questions are central to the aims of the ACEnano project.
What are the challenges of TERS?
A TERS microscope combines an atomic force microscope (AFM) with a Raman spectrometer. Raman is an optical technique, with a laser excitation source and a resulting light spectrum containing the chemical information. Most AFMs on the market are not designed for optical coupling, and either are not suitable for such coupling or impart significant drawbacks to the spectroscopy. When the company AIST-NT developed the SmartSPM™ AFM it did so with the clear purpose of allowing excellent optical coupling. As a result, AIST-NT (which was subsequently acquired by HORIBA) had to design a specific head and optical coupling system to enable Raman spectroscopy, both in a co-localized mode (i.e., making Raman measurements at the same position of the AFM scanning) and in the TERS mode (what we call NanoRaman™).
Another major consideration was how to make such a system as robust as possible. TERS is coupling nanoscale tip scanning, Raman spectroscopy, and plasmonic enhancements at the AFM tip – the end result being nanoscale chemical characterization. All three of those can present challenges on their own, so integrating them together required very careful design to ensure TERS could be useful in the laboratory, rather than simply becoming an interesting technical challenge for advanced optical engineers. Ensuring easy initial alignment, unparalleled stability, and continual feedback between Raman laser and tip were central to unlocking the power of TERS.
Another critical consideration is the probes (or tips). TERS relies on plasmonic enhancement at the tip – to achieve this you need the right probe (both physical design and its material). This was initially extremely challenging, but we now have tips that reliably and reproducibly work for TERS…so much so that we guarantee their performance.
Is the HORIBA TERS system the first of its kind?
The HORIBA TERS microscope is unique in that both the optical spectrometer and the AFM unit are built in the same factory in the north of France. It means that it is a single fully integrated system, rather than two separate components that are then joined together in the hope of achieving one of the most challenging spectroscopic measurements ever.
TERS has been considered and attempted for more than a decade, with some early limited success by individual research groups. The first attempts were based on trying to combine existing Raman spectrometers with existing AFMs, which certainly compromised performance, reliability, and robustness. What makes HORIBA’s proposal unique now is that it is a true turnkey TERS solution, guaranteed to offer sub-20 nanometer resolution, designed and built as a single system by a single manufacturer.
HORIBA Why is TERS beneficial for nanotoxicological studies
Is this the best kind of AFM-Raman system for this kind of work?
I have worked with many different AFMs over the last 15 years. The HORIBA AIST-NT system is certainly the best, offering a true turnkey TERS solution. You simply take your TERS probe, load it within the AFM head, and then you just have to work with the software to progress to NanoRaman™ measurements. Everything is automatic, so it is really quick to get to the point of making Raman measurements at the nanoscale. Visitors to our lab are always astonished to see how quickly we can move from loading the probe to actually making real TERS measurements!
The AFM also has a superb scanner that is drift-free, so you can record a sample overnight or over several days, in the same area, and it will have scanned the same area with virtually no drift at all. The system also has a “scanning objective” that was developed by HORIBA to achieve TERS. The Raman laser beam is passed through an objective in order to focus it on the tip but needs to be precisely aligned to the apex of the tip. With the HORIBA system, the objective incorporates a fast-feedback scanner which is used to first very accurately position the laser on the tip, and then it maintains the positioning all the time during the measurement. Without this, it would be impossible to maintain a good condition for TERS through long measurements.
What is unique about the TERS probes used within this system? How does that help with this type of work?
The TERS probes are critical to the success of a system. The probes are what give the enhancement of signal at the nanoscale, thus enabling chemical characterization with a spatial resolution well below the optical diffraction limit.
HORIBA offers two types of different probes: gold and silver. We all know that silver suffers from oxidation, so we deliver this probe with a passivation layer on top. This significantly slows the oxidation process, so that a single probe can be used successfully for seven weeks or more. Of course, the probes are designed with an optimal spring constant so you can use your system in contact and non-contact mode. Unlike other AFM measurements, a TERS measurement requires a dual analysis, first with the probe in contact with the sample, and then with the probe away from the sample (i.e., non-contact).
You have to swap between the two modes very quickly, within a few milliseconds, so you need to have the right spring constant over the probe to allow this, otherwise, you will damage your sample or break the tip. Getting this right is a really important consideration in experimental success!
Where will technology in this technique lead in the future? Where are things now, and what great opportunities do you see for this technology in the future?
15 years ago this technique was in its infancy, and I am very pleased to have such a microscope running routinely in my lab now. I think there's a big future for this technique, for instance in pharmaceutical laboratories and biophysics laboratories, to study bilayers and artificial membranes or artificial cells, which would be very useful areas for drug companies to understand. Drugs can penetrate cell membranes and target different organs or viruses. So TERS can be used to understand how drugs can target a tumor, for example – the particular strength of the technique is that you don’t need to use fluorescence probes, which potentially perturb the system you’re trying to study. With TERS you can achieve the chemical imaging at the nanoscale, without any perturbation.
There are also many successful measurements being made within the materials arena; substances such as carbon nanotubes (CNT), graphene, and transition metal dichalcogenides (TMDC) have great potential to enable clean energy, faster computing, new building capabilities, and exciting medical advances. Both TERS and TEPL (tip-enhanced photoluminescence, an analogous technique to TERS that probes the electronically excited states of the material) work extremely well for these materials.
Are people sceptical about TERS?
When TERS was first considered and tried, the potential of the technique was quickly understood, but the experimental methodology and instrument design were simply not ready. So whilst some successful measurements were made, they were few and far between, and difficult to replicate. There are many experts for Raman spectroscopy and many for AFM. TERS requires expertise in both, in addition to well-considered instrument design to enable NanoRaman™. I think some people have formed their opinion of TERS based on those very early attempts, but don’t realize the many developments and huge steps forward that have happened over the last few years. Maybe that is one of our jobs, as the instrument designer, to share our knowledge and expertise, and show the realistic state-of-the-art that TERS can readily achieve.

LabRAM Nano tip-enhanced Raman spectroscopy (TERS) system
Our involvement with major European projects such as ACEnano also helps to establish TERS as a recognized and useful technique, proving that it is now much more than just a technical challenge – it is a viable scientific method that answers real scientific questions.
What are the main advantages of TERS compared with existing techniques?
Currently, to study the bilayers or membranes or cells, scientists rely on fluorescence, confocal microscopes, or even top-technique nanoscale microscopy methods such as STORM-PALM microscopy. These microscopes rely on fluorescent probes, so you need to introduce a foreign element inside your cells or inside the materials you want to study. But in doing this, you perturb the very system you want to analyze. Fluorescence probes can even be toxic.
With TERS we rely simply on ‘seeing’ the vibrations of the molecules, by shining light on them. You don't have to introduce anything to do this - you take the sample, and you study it. It's easier and simpler, and experimentally more satisfactory.
Acknowledgment
The phospholipid bilayer research described in this article was assisted by funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No.720952.
About Pierre Burgos Ph.D.
 After graduating in Physical Chemistry at Nancy, Pierre Burgos completed his Ph.D. at the National Polytechnic Institute of Lorraine, France. His thesis work was performed mainly at the National Research Council of Canada in Ottawa where he developed a taste for scanning microscopy. After post-doctoral positions at the University of Arizona and the University of Sheffield, he secured a staff position at the Central Laser Facility at the Rutherford Appleton Laboratory in Oxfordshire where he was involved in supporting users in diverse spectroscopic measurements (FLIM, FRET, Raman Kerr gate, time-resolved techniques, etc). He joined HORIBA in July 2013 as a AFM-Raman product specialist, supporting customers and working on product development and new applications.
After graduating in Physical Chemistry at Nancy, Pierre Burgos completed his Ph.D. at the National Polytechnic Institute of Lorraine, France. His thesis work was performed mainly at the National Research Council of Canada in Ottawa where he developed a taste for scanning microscopy. After post-doctoral positions at the University of Arizona and the University of Sheffield, he secured a staff position at the Central Laser Facility at the Rutherford Appleton Laboratory in Oxfordshire where he was involved in supporting users in diverse spectroscopic measurements (FLIM, FRET, Raman Kerr gate, time-resolved techniques, etc). He joined HORIBA in July 2013 as a AFM-Raman product specialist, supporting customers and working on product development and new applications.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.