Epoxy resins cured with dianhydrides serve high-end polymer applications like adhesives, composites, molding compounds and powder coatings. The performance of the overall system is reliant upon the main ingredients of a system, e.g., resins and curatives.
Some properties, particularly cure behavior, are significantly dependent on the cure catalyst, one of the smallest ingredients in the formulation; this is also known as cure accelerator. This is equally true for epoxy formulations that utilize either dianhydrides or their monoanhydride cousins.
This article outlines the control achievable in cure behavior simply through catalyst choice.
DSC cure exotherm data is shown in Figure 1. This data is produced by heating unreacted formulation specimens at 10 °C/minute from ambient to 300 °C. The underlying epoxy-dianhydride formulation is outlined via the figures’ legend and the footnotes in this article.
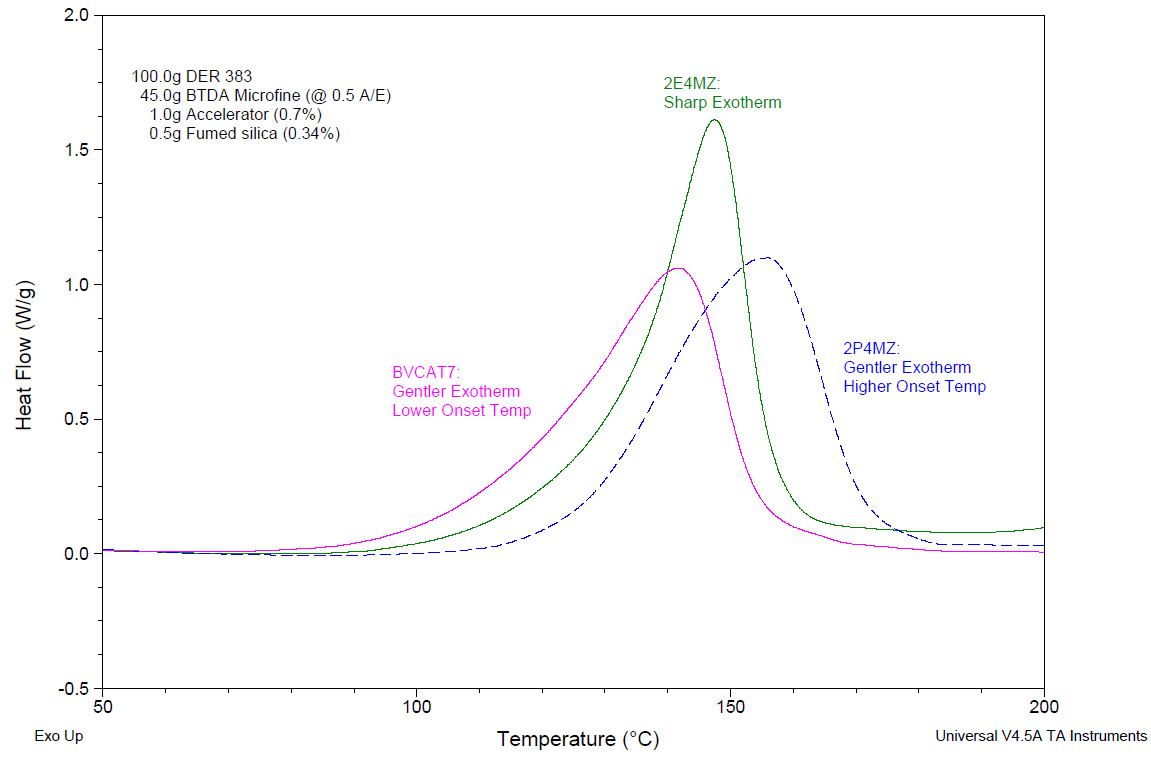
Figure 1. Effects of different catalysts on DSC cure exotherm data. Formulations contained liquid epoxy resin and BTDA (benzophenone tetracarboxylic dianhydride). Uncured formulations were subjected to DSC heating ramps @ 10 °C/min. Data from Mr. Nikola Bilic, Kansas Polymer Research Center, Pittsburg, KS.
A relatively sharp exotherm peak is demonstrated by the plot for 2E4MZ, an imidazole catalyst. This cure behavior can be helpful in applications where the quick development of strength is preferred after the cure temperature is attained. One case is high-temperature adhesives that are applied and cured in thin films.
Yet, such fast curing can also result in localized hot spots, which may cause degradation in the cured part and compromised performance.
In thicker applications, e.g., in the curing of high-performance composites, this would be a concern. Gentler cure behavior like those demonstrated for 2P4MZ (imidazole) and BVCAT-7 (ammonium salt) catalysts may be more suitable for these applications.
Their peak reaction rates are relatively mild, as seen in the figure; this is indicated by their modest peak exotherms. The catalyst BVCAT-7 can be chosen specifically for applications where such a moderate exotherm profile is suitable, but the process requires a lower cure temperature.
For instance, some mold materials in composite applications may be sensitive to temperature. The overall shape of the exotherm curve is just like the curve for 2P4MZ, yet the plot for BVCAT-7 points to a lower temperature for cure onset.
On the other hand, 2P4MZ may be more suitable in instances where high cure temperatures can be tolerated, but the intricacy of the mold may require slower fills for even warm resin mixes.
2P4MZ’s higher temperature for cure activation can be advantageous in those instances, as it decreases the risk of gelation during the filling process.
There are a number of catalyst types available for dianhydride-based epoxy systems. If a formulation is near to meeting the challenges of a demanding application, a deeper understanding of catalyst behavior can be the missing piece for achieving success.
Footnotes
- DER383 = D.E.R.TM 383 liquid epoxy resin, EEW 182g/eq.
- BTDA = JAYHAWK BTDA® Microfine.
- 2E4MZ = Imicure® 2E4MZ = 2 ethyl 4 methyl imidazole
- 2P4MZ = Curezol® 2P4MZ = 2 phenyl 4-methyl imidazole
- BVCAT-7 is a quaternary ammonium salt catalyst available from Broadview Technologies.
- BTDA is a trademark of Jayhawk Fine Chemicals Corporation.
- D.E.R. is a trademark of Olin Corporation.
- Imicure and Curezol are trademarks of Evonik Corporation.

This information has been sourced, reviewed and adapted from materials provided by Jayhawk Fine Chemicals Corporation.
For more information on this source, please visit Jayhawk Fine Chemicals Corporation.