|
Trauma, degeneration and diseases often make surgical repair or replacement necessary. When a person has a joint pain the main concern is the relief of pain and return to a healthy and functional life style. This usually requires replacement of skeletal parts that include knees, hips, finger joints, elbows, vertebrae, teeth, and repair of the mandible. The worldwide biomaterials market is valued at close to $24,000M. Orthopaedic and dental applications represent approximately 55% of the total biomaterials market. Orthopaedics products worldwide exceeded $13 billion in 2000, an increase of 12 percent over 1999 revenues. Expansion in these areas is expected to continue due to number of factors, including the ageing population, an increasing preference by younger to middle aged candidates to undertake surgery, improvements in the technology and life style, a better understanding of body functionality, improved aesthetics and need for better function.
What are Biomaterials?
Biomaterial by definition is “a non-drug substance suitable for inclusion in systems which augment or replace the function of bodily tissues or organs”. From as early as a century ago artificial materials and devices have been developed to a point where they can replace various components of the human body. These materials are capable of being in contact with bodily fluids and tissues for prolonged periods of time, whilst eliciting little if any adverse reactions.
Historical Development of Biomaterials
Some of the earliest biomaterial applications were as far back as ancient Phoenicia where loose teeth were bound together with gold wires for tying artificial ones to neighbouring teeth. In the early 1900’s bone plates were successfully implemented to stabilise bone fractures and to accelerate their healing. While by the time of the 1950’s to 60’s, blood vessel replacement were in clinical trials and artificial heart valves and hip joints were in development.
Design Factors for Biomaterials
Even in the preliminary stages of this field, surgeons and engineers identified materials and design problems that resulted in premature loss of implant function through mechanical failure, corrosion or inadequate biocompatibility of the component. Key factors in a biomaterial usage are its biocompatibility, biofunctionality, and availability to a lesser extent. Ceramics are ideal candidates with respect to all the above functions, except for their brittle behaviour.
Implant Materials
It has been accepted that no foreign material placed within a living body is completely compatible. The only substances that conform completely are those manufactured by the body itself (autogenous) and any other substance that is recognized as foreign, initiates some type of reaction (host-tissue response). The four types of responses, which allow different means of achieving attachment of implants to the muscular skeletal system, are given in Figure 1.
|
|
|
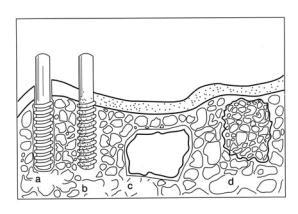
Figure 1. Classification of biomaterials according to their bioactivity (a) bioinert alumina dental implant, (b) bioactive hydroxyapatite [Ca10(PO4)6(OH)2] coating on a metallic dental implant, (c) surface active bioglass and (d) bioresorbable tricalcium phosphate ([Ca3(PO4)2] impant.
|
Biomaterials Classifications
When a synthetic material is placed within the human body, tissue reacts towards the implant in a variety of ways depending on the material type. The mechanism of tissue interaction (if any) depends on the tissue response to the implant surface. In general, there are three terms in which a biomaterial may be described in or classified into representing the tissues responses. These are bioinert, bioresorbable, and bioactive, which are well covered in range of excellent review papers.
Bioinert Biomaterials
The term bioinert refers to any material that once placed in the human body has minimal interaction with its surrounding tissue, examples of these are stainless steel, titanium, alumina, partially stabilised zirconia, and ultra high molecular weight polyethylene. Generally a fibrous capsule might form around bioinert implants hence its biofunctionality relies on tissue integration through the implant (Figure 1a).
Bioactive Biomaterials
Bioactive refers to a material, which upon being placed within the human body interacts with the surrounding bone and in some cases, even soft tissue. This occurs through a time – dependent kinetic modification of the surface, triggered by their implantation within the living bone. An ion – exchange reaction between the bioactive implant and surrounding body fluids – results in the formation of a biologically active carbonate apatite (CHAp) layer on the implant that is chemically and crystallographically equivalent to the mineral phase in bone. Prime examples of these materials are synthetic hydroxyapatite [Ca10(PO4)6(OH)2], glass ceramic A-W and bioglass® (Figure 1b and c)).
Bioresorbable Biomaterials
Bioresorbable refers to a material that upon placement within the human body starts to dissolve (resorbed) and slowly replaced by advancing tissue (such as bone). Common examples of bioresorbable materials are tricalcium phosphate [Ca3(PO4)2] and polylactic–polyglycolic acid copolymers. Calcium oxide, calcium carbonate and gypsum are other common materials that have been utilised during the last three decades (Figure 1d).
|