Introduction Zinc oxide has many properties including photoconductivity, piezoelectricity, pyroelectricity and fluorescence. Since ZnO has a wide direct band-gap of 3.37 eV, much attention has been paid to the development of ZnO light-emitting devices in the blue to UV region. The advantage of ZnO is its large exciton binding energy of about 60 meV. An excitonic laser action was observed at room temperature from ZnO with high crystalline quality [1-4]. Many different techniques have been used to obtain ZnO exhibiting UV emission [5-8]. We have recently developed a new ZnO crystal growth method using the thermite reaction combined with electric current heating [9]. When a certain current flowed through the bar on which Al powder was placed, a cluster of ZnO crystals grew on the Al powder. The aim of the present work is to investigate the influence of oxygen partial pressure in the growth atmosphere on the structures and luminescence properties of the clusters grown by the electric current heating with thermite reaction. Experimental The ZnO powder (Soekawa Chemicals, 99.999% purity) was pressed uniaxially at 20 MPa, sintered at 1000°C for 3 h in air and then cut into a rod shape piece of 15 mm × 1 mm × 1 mm. The relative density of the resultant ceramics was approximately 90%. The outer edges of each rod were electroded using a Pt paste. Al powder (Soekawa Chemicals, 99.9% purity, 150 mesh) was placed at the center of the rod. The sample was placed in a glass tube (50 mm in diameter, 300 mm in length) with gas flow. Oxygen partial pressure (Po2) was controlled using an Ar/O2 gas mixture and monitored using ZrO2 oxygen sensor. A direct current flowed through the sample using a regulated dc power supply. The morphology of the sample was observed using a digital microscope (KEYENCE, VH-7000). For photoluminescence (PL) measurements, He-Cd laser (325 nm) (Kimmon Electric, IK-3301R-G) was used for the excitation. Results and Discussion When a current of 70 A/cm2 flowed, the bar glowed by joule heating, allowing the thermite reaction to take place in the Al powder. After several seconds, a cluster of ZnO crystals grew on the Al powder. Figure 1 shows the ZnO cluster on the bar after electric current heating with thermite reaction. The cluster grew under the conditions of a current density of 70 A/cm2 and Po2 from 9 to 30 kPa. In Po2 of 8 kPa or less, the thermite reaction did not occur. At Po2 = 10 kPa (b), the ZnO cluster had a spherical shape and its length was about 0.3 mm. At Po2 = 20 kPa (c), the form became a stem shape. At Po2 = 30 kPa (d), the cluster again had a spherical shape. In Po2 over 30 kPa (e), Al was scattered by the intense reaction. | 



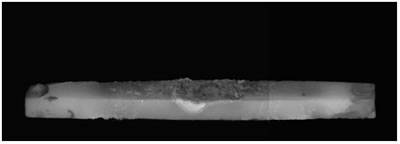
| | Figure 1. Photographs of the cluster of ZnO crystals on a ZnO ceramic bar after electric current heating with thermite reaction. The cluster grew under a current density of 70 A/cm2 in several oxygen partial pressures. (a) Before heating, (b) Po2 = 10 kPa, (c) Po2 = 20 kPa, (d) Po2 = 30 kPa, (e) Po2 = 40 kPa. | Figure 2 shows the oxygen partial pressure dependence of the ZnO cluster length. With increasing Po2 over 9kPa, the length of the cluster increased, showed the maximum (~2.7mm) at 20kPa, and decreased. It was found that the length of the cluster could be controlled by changing the oxygen partial pressure under the electric current heating process. The change in the length of the cluster was probably caused by the different oxidation rate of the Al powder. In low Po2, the long cluster did not grow because of the poor reactivity of the Al powder. In high Po2, the oxidation of Al occurred too fast for the cluster to grow. | 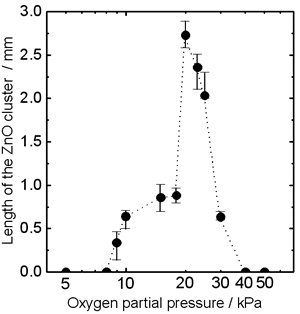
| | Figure 2. Oxygen partial pressure dependence of the length of the ZnO cluster. The cluster grew under a current density of 70 A/cm2. | Figure 3 shows typical PL spectra from the top of the ZnO clusters grown under various oxygen partial pressures. The PL strongly depended on the Po2. The PL peak of the clusters grown at Po2 = 10 (a) and 15 kPa (b) showed only the well-known visible emissions at approximately 2.4 eV. Intense UV emission was observed from the cluster grown at Po2 = 20 kPa (c). The clusters grown at Po2 = 25 (d) and 30 kPa (e) showed UV and visible emissions. | 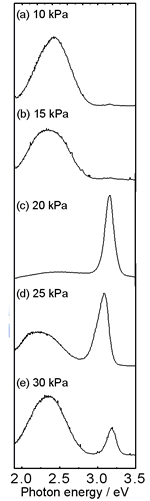
| | Figure 3. PL spectra from the top of the ZnO cluster grown in various oxygen partial pressures (a) 10 kPa, (b) 15 kPa, (c) 20 kPa, (d) 25 kPa, (e) 30 kPa. The spectra were measured at room temperature using He-Cd laser (325 nm) as an excitation source. | Figure 4 shows the oxygen partial pressure dependence of the relative intensity of UV and visible emission. In Po2 from 9 to 18 kPa, the visible emissions were dominant. At 20 kPa the intensity of visible emission abruptly decreased to approximately zero and the intensity of UV emission increased remarkably. With increasing Po2 over 20 kPa, the intensity of UV emission decreased. The strongest UV emission was observed from the cluster grown at Po2 = 20 kPa. | 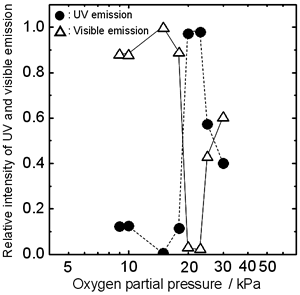
| | Figure 4. The oxygen partial pressure dependence of the relative intensity of UV and visible emission. The cluster grew under a current density of 70 A/cm2. | The growth mechanism of ZnO cluster is considered to be as follows. First, thermite reaction occurs due to elevated temperature by electric current heating. And Zn vapor is generated on the surface of the ZnO ceramic bar due to high temperature and reductive atmosphere, diffuses through the opening in the Al powder and is oxidized immediately after it reaches the Al powder surface. Then, the generated ZnO seals the opening, and the pressure of Zn gas in the Al powder is increased. After several seconds, a spout of the Zn gas occurs. Finally, the ZnO cluster is grown on the Al powder. The growing part is considered to be the top of the ZnO cluster. This consideration can be deduced from the following. The temperature of the top of the longer ZnO cluster is lower during the growth, since the distance from the top to the heated bar is longer. The number of thermally generated defects decreases with decreasing temperature. Therefore the top of the longer ZnO cluster has higher crystallinity. The stronger UV emission observed at the top of the longer cluster supports the above consideration. Conclusions The effect of oxygen partial pressure on luminescence of the ZnO cluster grown by electric current heating with thermite reaction was investigated. The cluster grew in the atmosphere with oxygen partial pressure from 9 to 30 kPa. The maximum length of the cluster was obtained at Po2 = 20 kPa. The maximum UV emission was also observed from the cluster grown at Po2 = 20 kPa. From these results, the growing part is considered to be the top of the cluster. Acknowledgements This study was supported partially by Grants for Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (No. 50381) and 21st Century COE Program “Creation of Hybridized Materials with Super-Functions and Formation of International Research & Education Center” from the Ministry of Education, Culture, Sports, Science and Technology, Japan. References 1. P. Zu, Z.K. Tang, G.K.L. Wong, M. Kawasaki, A. Ohtomo, H. Koinuma and Y. Segawa, “Ultraviolet Spontaneous and Stimulated Emissions from ZnO Microcrystallite Thin Films at Room Temperature”, Sol. Stat. Commun., (b) 103 (1997) 459-463. 2. D.M. Bagnall, Y.F. Chen, Z. Zhu, T. Yao, S. Koyama, M.Y. Shen and T. Goto, “Optically Pumped Lasing of ZnO at Room Temperature”, Appl. Phys. Lett., 70 (1997) 2230-2232. 3. S. Cho, J. Ma, Y. Kim, Y. Sun, G.K.L. Wong and J. B. Ketterson, “Photoluminescence and Ultraviolet Lasing of Polycrystalline ZnO Thin Films Prepared by the Oxidation of the Metallic Zn”, Appl. Phys. Lett., 75 (1999) 2761-2763. 4. M.H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, “Room-Temperature Ultraviolet Nanowire Nanolasers”, Science, 292 (2001) 1897-1899. 5. D. Nezaki, T. Okamoto and M. Takata, “Structure and Photoluminescence of ZnO Crystal Grown by Electric Current Heating Method”, Key Eng. Mater., 228-229 (2002) 241-244. 6. H. Nanto, T. Minami and S. Takata, “Photoluminescence in Sputtered ZnO Thin Films”, Phys. Stat. Sol., A65 (1981) K131-K134. 7. R. D. Vispute, V. Talyansky, S. Choopun, R. P. Sharma, T. Venkatesan, M. He, X. Tang, J. B. Halpern, M. G. Spencer, Y.X. Li, L. G. Salamanca-Riba, A. A. Iliadis and K. A. Jones, “Heteroepitaxy of ZnO on GaN and its Implications for Fabrication of Hybrid Optoelectronic Devices”, Appl. Phys. Lett., 73 (1998) 348-350. 8. S. Bethke, H. Pan and B. W. Wesseis, “Luminescence of Heteroepitaxial Zinc Oxide”, Appl. Phys. Lett., 52 (1988) 138-140. 9. K. Minato, D. Nezaki, T. Okamoto and M. Takata, “Growth Conditions and Luminescence of ZnO Crystals Grown by Electric Current Heating with Thermite Reaction”, Key. Eng. Mater., 248 (2003) 95-98. Contact Details |