DOI :
10.2240/azojomo0288
Aug 19 2009
Makoto Nanko
Copyright AD-TECH; licensee AZoM.com Pty Ltd.
This is an AZo Open Access Rewards System (AZo-OARS) article distributed under the terms of the AZo-OARS https://www.azom.com/oars.asp which permits unrestricted use provided the original work is properly cited but is limited to non-commercial distribution and reproduction.
AZojomo (ISSN 1833-122X) Volume 6 August 2009
Topics Covered
Abstract
Keywords
Introduction
Definition and Category of Hybrid Materials Described in the Literature
Hybrid Composite Materials
Inorganic/Organic Hybrids
Hybridization of Functions
New Classification of Hybrid Materials
Summary
Acknowledgements
References
Contact Details
Abstract
A new systematic definition and the category of hybrid materials are proposed in this paper. The categories are (1) structurally-hybridized materials, (2) materials hybridized in chemical-bond, and (3) functionally-hybridized materials. Different categories of hybrid materials can be designed using different concepts of hybridization. A systematic design concept for of hybridization of functions is required for the development of new functionally-hybridized materials with superior properties and new functions.
Keywords
Hybrid Materials, Composites, Chemical-Bond, Function
Introduction
Since Toyota’s Pryus hybrid automobile appeared in 1997, hybrid cars have become common. Recently, the word "hybrid" has also been used in materials science and engineering. While the terms "hybrid" and "hybrid materials" have been used to express materials that have been mixed with different materials, materials consisting of different materials are conventionally called "composites". In many cases, when the word "hybrid" was used, the term was not defined, and the difference between hybrid materials and composites has also not been clarified.
In this paper, a new systematic definition and category of hybrid materials are proposed in order to understand the various types of hybrid materials and composites, and to establish new concept of materials design. The new category of hybrid materials is based on the concept of, "what has been hybridized?"
Definition and Category of Hybrid Materials Described in the Literature
Yamamoto et al. [1] defined hybrid materials as mixtures of two or more materials with new properties created by new electron orbitals formed between each material, such as covalent bond between polymer and silanol molecular in inorganic/organic hybrids (described later). Makishima [2] categorized substances into three materials by their chemical-bond modes, i.e., metals, organic materials and their polymers, and ceramics. He also defined hybrid materials as mixtures of two or more materials with newly-formed chemical-bonds. His categorization of hybrid materials and their related materials was proposed as follows:
- Composites: Mixture of materials consisting of matrix and micron-level dispersion
- Nanocomposites: Sub-micron level mixture of similar kinds of materials
- Hybrids: Sub-micron level mixture of different kinds of materials
- Nanohybrids: Atomic or molecular level mixture of different materials with chemical-bonds between their different materials
Makisima explained that the difference between hybrids and nanohybrids was not so obvious, and that nanocomposites include hybrids and nanohybrids in many cases. Gómez-Romero and Sanchez [3] defined hybrid materials as organic-inorganic hybrid materials or inorganic-biomaterials. They also mentioned that the characteristic scale of hybrid materials was less than 103 nm. They did not provide a strict definition of hybrid materials, and did not mention the formation of new electron orbitals or chemical bonds. In the Makisima classification and the Gomez-Romero & Sanchez definition, mixtures of materials were focused at the viewpoint of the characteristic scale and materials category. Their definitions of "hybrid materials" required an atomic or nanometer-level mixture of materials. Figure 1 shows a classification of materials by different scale levels, as proposed by the Materials Science Society of Japan [4].
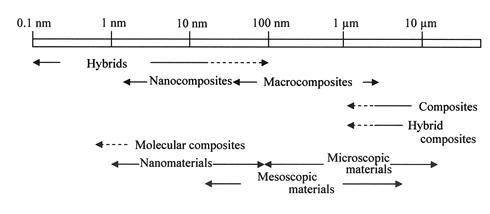
Figure 1. Classification of Materials at Different Scale Levels [4].
On the other hand, Ashby [5] defined hybrid materials as, "a combination of two or more materials in a predetermined geometry and scale, optimally serving a specific engineering purpose". Hagiwara and Suzuki [6] described hybrid materials as an intentional combination of two or more materials, complimenting each other to have super-functions or new functions which component materials did not possess. According to their criterion, the difference between hybrid materials and composites lied in their functions and/or properties. Hybrid materials must have superior functions or properties comparing to traditional composites. In this paper, the author proposes systematic criterion for hybrid materials from the point of view of the purpose of hybridization. In the present proposal, hybrid materials can be distinguished into three categories:
- Structurally-hybridized materials,
- Materials hybridized in chemical-bond, and
- Functionally-hybridized materials,
Hybrid Composite Materials
A hybrid composite material is a combination of "hybrid" and "composite" [7]. This material is simply a hybridization of composite materials, for example, composites reinforced with two or more types of fibers or a laminar composite material consisting of fiber-reinforced metals and thin foil metals. The meaning of "hybrid" in hybrid composite materials is the hybridization in macroscopic structure in the metallographic scale.
In some cases relating to functional materials, the term "hybrid materials" may be preferable. Because structural composites such as carbon/carbon composites and metallic matrix composites have been very popular, the term "composites" may sound conventional for researchers of functional materials.
The hybrid materials described in this section are composites with a hybridization of macroscopic mixtures. Properties of these materials can be understood from the combination of properties of the component materials, for example, the rule of mixture. These materials should be termed "structurally-hybridized materials" because hybridization of the macroscopic structure is the purpose of the combination or mixture of materials. Nanocomposites, composites with a characteristic scale less than a micro-meter, have excellent properties when compared to macroscopic composites. Their fine microstructure or grain boundary effects are responsible for the excellent properties of nanocomposites. Nanocomposites are intended to have a nanometer-scale structure of mixing. For this reason, nanocomposites are a kind of structurally-hybridized materials. However, some nanocomposites have excellent properties based on the particular chemical-bonds at the interface between the component materials. In this case, the purpose of the mixture is not the creation of composite structure, and instead is the creation of new chemical-bonds.
Inorganic/Organic Hybrids
Inorganic/organic hybrids have received a great deal of attention from many different fields, and have been a definite hot topic in materials science and engineering. Suyama [8] proposed three types of inorganic/organic hybrids, categorizing them on the basis of structural differences in the hybridization of inorganic and organic materials. The first example of inorganic/organic hybrids is organic-modified silicates fabricated by sol-gel processing. The organic-modified silicates, as shown in Fig. 2 (a), have excellent mechanical properties due to strong covalent bond between silica and organic molecules mixed in molecular scale [8]. Hybrid materials fabricated in such a manner not only include dispersed silica clusters into polymer materials, but also are characterized by the particular chemical-bonds between silica and organic molecules, in contrast with traditional composites. Nowadays, there are many types of inorganic/organic hybrids. A clay/polymer hybrid is one example of an inorganic/organic hybrid material, as shown in Fig. 2 (b). Strong chemical-bond between silicate monolayers and polymer molecules offers much improved mechanical properties and lower gas permeability than those of polymer materials.
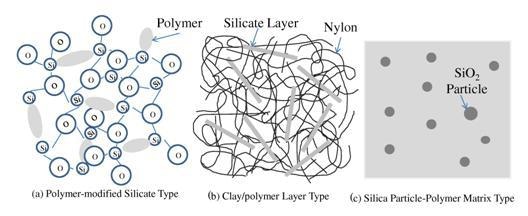
Figure 2. Morphology in Inorganic/Organic Hybrids [8].
As shown in Fig. 3, the structural difference between hybrid materials and nanocomposites consisting of clay and polymer materials is very clear [9].
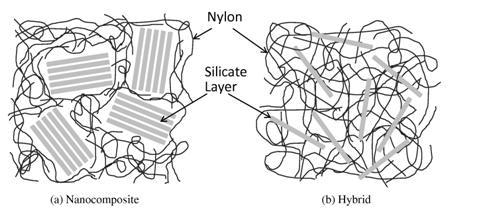
Figure 3. Differences between Inorganic-Organic Hybrids and Inorganic-Organic Nanocomposites; Example of a Clay-Nylon System [9].
Inorganic/organic hybrids are not a simple combination of inorganic and organic materials. They are a new category of materials, possessing particular chemical-bonds between inorganic and organic materials, which is different from characteristic chemical-bond. Some inorganic/organic hybrids have excellent thermal resistance over 1000ºC [10] and they are no longer categorized into organic materials in the general sense.
Organic molecules involving metallic atoms or inorganic metal oxides through chemical-bond are so-called hybrid molecules [11]. They are regarded as precursors of inorganic/organic hybrids. Some hybrid molecules show unique catalytic activity, optical properties, electric properties, and so on.
In inorganic/organic hybrids, particular chemical-bond between organic and inorganic species produces excellent properties in macroscopic scale.
The development of particular chemical-bond between organic and inorganic molecules is analogous to the formation of hybrid orbitals between different kinds of molecules or atoms. Yamada et al. [1] propose "hybrid" materials by expanding the concept of inorganic/organic hybrid materials.
Hybrid materials are based on the interactions between different molecules that appear similar to the hybrid orbital or orbital conversions between different kinds of molecules and atoms. Hybrid materials are regarded as boundary-area materials among organic, inorganic and metallic materials and should be distinguished from structurally-hybridized materials since the concept of hybridization is completely different from that of structural hybrids. Niizeki [12] also describes hybrid materials as involving two or more kinds of chemical-bonds created by "hybridizing" two or more monolithic materials, and as a result hybrid materials have superior performance and/or new functions in comparison with their component monolithic counterparts. In this paper, these hybrid materials are referred to as "materials hybridized in chemical-bond".
Hybridization of Functions
Photocatalysts with absorbents, such as TiO2/graphite, TiO2/hydroxyapatite and TiO2/SiO2, have much better decomposition capacity of organic compounds than monolithic photocatalysts [13-15]. Although TiO2 photocatalysts have excellent photocatalytic properties, they cannot absorb molecules to be decomposed. As a result, monolithic photocatalysts have a low decomposition rate of organic molecules. In order to overcome this disadvantage, the hybridization of photocatalysts and absorbents has been proposed. These types of photocatalysts are called "hybrid photocatalysts".
Intelligent materials, or smart materials, can be defined as structural materials featuring a sensing function, self-recovery function, and memory function. They are usually composite materials [16, 17]. Hybridization of ionic conductive ceramics and electron conductive materials leads to the creation of selective gas permeable solids, such as hydrogen or oxygen [18, 19] and in such material combinations, the development of particular chemical-bond does not occur.
Rather the combination of two or more functions causes new or superior functions to be created. Therefore, the creation of a new functions or super functions can be established by combining the hybridization of the functions of each material. Here, these types of hybrid materials are defined as "functionally-hybridized materials".
When a certain property of a material provides its users benefits (sometimes negative effects), the property can be defined as a function of the material. In this section, the functions of materials will be categorized. Table 1 shows the categories of material functions proposed.
Functions (2) to (6) are combinations of the primary function (Function (1)), and so can be called high-order functions. Functionally-hybridized materials can be defined as composites with artificially created functions, created by sophisticatedly mixing two or more materials. The present paper must consider how to create superior functions in functionally-hybridized materials by mixing two or more materials. Understanding function creation for functionally-hybridized materials is essential in designing functionally-hybridized materials.
Table 1. Classification of Material Functions.
|
No.
|
Functions
|
Remarks
|
|
(1)
|
Single Functions
|
Mass, Strength, Conductivity, Corrosion resistance, etc.
|
|
(2)
|
Secondary Functions
|
Thermoelectric functions, Specific strength, Heat capacitance etc.
|
|
(3)
|
Sensing
|
Properties changes by corresponding input signals
|
|
(4)
|
Chemical Reaction Functions
|
Catalysis, Selective permeation, etc.
|
|
(5)
|
Intelligences
|
Self-healing, Shape memory, Memory function, etc.
|
|
(6)
|
Environmental Functions
|
Anti-environmental impacts, Recycling performance, etc.
|
|
(7)
|
Bio/human-Oriented Functions
|
Toxicity, Bio-activity, Color, Tactile impression, etc.
|
|
(8)
|
Application-Specific Functions
|
Cutting performance for cutting tools
|
Although material’s users determine the functions of materials on the basis of materials properties, the actions of the hybridization of materials and the mechanisms to create their functions using hybridization are a concern of materials science. Tables 2 and 3 list the actions of hybridization and their mechanisms to create material functions.
Table 2. Action in Mixing to Create Functions of Functionally-Hybridized Materials.
|
No
|
Action
|
Remarks
|
|
(a)
|
Averaging
|
Create average properties by mixing two or more materials
|
|
(b)
|
Supplement/ Strengthening
|
Improve/strengthen functions of a material by mixing other materials
|
|
(c)
|
Addition
|
Add different functions into a material by mixing with other materials
|
|
(d)
|
Collaboration
|
Create new functions by collaborating different functions in different materials
|
|
(e)
|
Creation
|
Create new functions by mixing different materials
|
Table 3. Mechanisms to Create Functions in Functionally-Hybridized Materials.
|
No
|
Mechanism
|
Remarks
|
|
(a)
|
Interface Effect
|
Rectification: Schottokey diodes
|
|
(b)
|
Pinning Effect
|
Pining of dislocation: Precipitation hardening alloys, Pinning of magnetic field: Superconducting oxides
|
|
(c)
|
Quantum Effect
|
Nanocomposites
|
|
(d)
|
Morphological Effect
|
Light scattering and reflection: Photonic crystals, etc.
|
|
(e)
|
Combination Effect
|
New function created by a combination of functions
|
|
(f)
|
Multiplier Effect
|
Function enhancement caused by interaction between each component
|
In conventional composites, that is, structurally-hybridized materials, (a) averaging and (b) supplement/strengthening are usually applied. Intelligent materials and smart materials are classified into the (c) addition of functions. They are types of functionally-hybridized materials. Some hybridization methods of materials that create new functions are given by (d) collaboration and (e) creation.
Materials hybridized in chemical-bond have a unique electron state that is created by mixing materials, and they have the potential to have unique functions. On the other hand, in functionally-hybridized materials with Action (e) collaboration, mixing the different properties of each material creates new functions. Therefore, the formation of a new chemical-bond state by mixing materials is not preferred. For example, a p-n junction gives a rectification behavior by the particular band structure at the interface. However, the interfacial chemical-bond is not major bond in a p-n junction. That is, a p-n junction is not a material hybridized in chemical-bond; it is actually a functionally-hybridized material.
New Classification of Hybrid Materials
As mentioned before, there are three categories of hybridization of materials, that is, hybrid materials. The proposed categorization of hybrid materials according to Makishima is listed as follows:
- Structurally-Hybridized Materials (Composites)
- Materials Hybridized in Chemical-Bond, and
- Functionally-Hybridized Materials
Figure 4 shows the relationship between the three different categories of hybrids. Some hybrid materials may be difficult to clearly assign to a certain type in the categories proposed here.
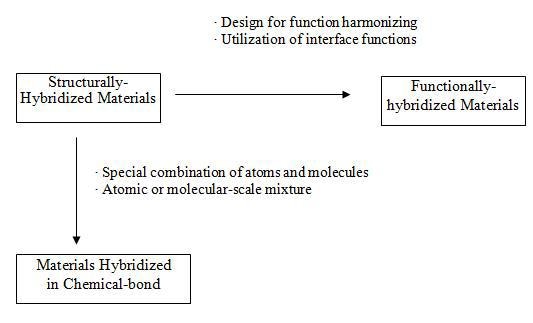
Figure 4. Relationship between Structurally-Hybridized Materials, Materials Hybridized in Chemical-Bond and Functionally-Hybridized Materials.
For example, there are hybrids that have the features of both materials hybridized in chemical-bond and functionally-hybridized materials; synergy ceramics [20, 21] is typically a mixture of different hybridization concepts that are described here. The concept of synergy ceramics is the simultaneous control of structural elements covering multiple size-scales with hyper-organized structure control, resulting in the harmonization of several trade-off functions such as strength and toughness. The concept of synergy ceramics involves the hybridization of functions to achieve harmonizing tread-off functions by controlling the material structure ranging from atomic level to macroscopic scale. The concept of synergy ceramics is simply mixtures of the three different hybridization concepts described here.
The most important aspect of the proposed categorization of hybrid materials is that each different category of hybrids can be designed as a basis for a different hybridization concept. Structurally-hybridized materials are based on the rule of mixture. Materials hybridized in chemical-bond can be designed by using a quantum chemistry computer simulation concerning the electron orbital. Functionally-hybridized materials should be created as a basis of the concept to harmonize each function of component material, resulting in new functions or super functions. Unfortunately, the concept of the hybridization of functions has not been established. The systematic concept to design functionally-hybridized materials will be a new topic of study in materials science and engineering.
Summary
A new systematic definition and the categorization of hybrid materials were proposed in this paper. The categories are (1) structurally-hybridized materials, (2) materials hybridized in chemical-bond, and (3) functionally-hybridized materials. Different categories of hybrid materials can be designed using different concepts of hybridization.
A systematic design concept for functionally-hybridized materials, how to create function of materials by combination of two or more function of component materials by mixing them, is required for the development of new functionally-hybridized materials with superior properties and new functions.
Acknowledgements
The author expresses gratitude to the Japanese government for partially supporting this work through the 21st Century Center of Excellence (COE) Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
References
- A. Yamada, H. Sasabe, Y. Osada and Y. Shiroda, I. Concepts of Hybrid Materials, Hybrid Materials –Concept and Case Studies, ASM International, OH, USA, 1989.
- A. Makisima, "Possibility of Hybrids Materials", Ceramic Japan, 39, (2004) 90-91. [in Japanese]
- P. Gómez-Romero and C. Sanchez, Functional Hybrid Materials, ed. by P. Gómez-Romero and C. Sanchez, Wiley-VCH Verlag GmbH & Co., (2004) 1-6.
- Materials Science Society of Japan, Molecular Hybridization and Hybrid Materials, Composite System in Materials, Shokabo Publishing Co., Tokyo, Japan (1993) 336-343.
- M. F. Ashby and Y. J. M. Bréchet, "Designing Hybrid Materials", Acta Mater., 51 (2003) 5801-5821.
- Y. Hagiwara and H. Suzuki, Fracture Mechanics, Ohmsha, Tokyo, Japan, (2000) 135-138. [in Japanese]
- M. Uemura, Hybrid Composites, Ed. by M. Uemura and H. Hukuda, CMC Publishing Co., Tokyo, Japan, (2002) 1-9. [in Japanese]
- Y. Suyama, "Research and Development of Organic-Inorganic Nanohybrids Materials", Ceramics Japan, 39 (2004) 92-93. [in Japanese]
- A. Usuki, "Organic-Inorganic Hybrid Materials", Expected Materials for the Future, 1[5] (2001) 6-13. [in Japanese]
- K. Obata, "Silicon-Containing Organic/Inorganic Hybrid Materials", Network Polymers, 25 [4] (2004) 34-43. [in Japanese]
- Materials Science Society of Japan, Composite System in Materials, Shokabo, Tokyo, Japan, (1993) 331-336. [in Japanese]
- N. Niizeki, "What are Hybrid Materials?", Sensor Tech., 6 [2] (1986) 42-44. [in Japanese]
- K. Ichimura, Fundamental and Applications of Harmonized Molecular Materials, CMC Publishing, Tokyo, Japan, (2004) 1-3. [in Japanese]
- T. Nonami, "New Progress of Visible Light-Type Photocatalysts and Photocatalytic Technology: Hybridization of Apatite and Titania Photocatalysts", Industrial Materials, 50[7] (2002) 60-64. [in Japanese]
- S. Akiyama and H. Taoda, Photocatalyst and its Related Technology, Nikkan Kogyo Sinbun Sya, (2000) 37-48. [in Japanese]
- P. F. Gobin, G. Guénin, M. Morin, M. Salvia and J. Tatibouet, "Smart Materials: A Future for Composites", J. Intell. Mater. Syst. Struct., 7 (1996) 353-357.
- J. Tani, T. Takagi and J. Qiu, "Intelligent Material Systems: Application of Functional Materials", Appl. Mech. Rev., 51 (1998) 505-521.
- K. Huang, M. Schroeder and J. B. Goodenough, "Oxygen Permeation though Composite Oxide-Ion and Electronic Conductors", Electrochem. Solid-State. Lett., 2 (1999) 375-378.
- K. Kobayashi and T. Tsunoda, "Oxygen Permeation and Electrical Transport Properties of 60 vol% Bi1.6Y0.4O3 and 40 vol% Ag Composite Prepared by the Sol-Gel Method", Solid State Ionics, 175 (2004) 405-408.
- M. Shimada, "Synergy Ceramics with Hyper-Organized Structure Control", Materia Japan, 36 (1997) 950-953. [in Japanese]
- A. Tsuge, "The Present Status of the Synergy Ceramics Project", Key Eng. Mater., 247 (2003) 447-454.
Contact Details
Makoto Nanko
Nagaoka University of Technology
1603-1, Kamitomioka
Nagaoka, Niigata 940-2188
JAPAN
E-mail: [email protected]
This paper was also published in print form in "Advances in Technology of Materials and Materials Processing", 11[1] (2009) 1-8.