|
Modern dental practice has become very dependent on its materials, such that the dentist's greatest challenge is choosing the right combinations of them for the benefit of their patients.
Metallic Fillings
Silver Amalgams
For over 150 years, silver amalgam has been used to fill the cavities made by dentists during the removal of dental decay from teeth. When pure silver (melting point 961°C) is mixed with mercury (mp -39°C) it produces a paste of slowly forming intermetallic compounds. When this is packed into the cavity at body temperature (37°C), the intermetallic compounds interlock and the amalgam hardens. However, setting is accompanied by a considerable expansion, and 100 years ago it was discovered that this can be controlled by adding tin to the silver. Unfortunately, this produces corrodible tin-mercury intermetallic phases, and their loss can cause breakdown of the filling.
By adding copper, the tin-mercury phase is eliminated and modern dental amalgams are made by mixing silver-tin-copper alloy powder with mercury. This results in fillings that resist both the mechanical and chemical onslaughts within the mouth for many years.
Although the amount of mercury lost from such fillings is like the contamination of a drink by a drowning midge, those determined to deny the benefits of having usefully restored teeth have over emphasised the risk, and this has generated a search for an alternative, metallic, mercury-free, filling material.
Alternatives to Silver Amalgams
Gallium (mp 30°C) has been combined with indium and tin to produce an alloy that is molten at normal room temperature, and when this is mixed with powdered silver-tin-copper it produces a paste that sets. However, packing this paste has proved to be a challenge, and the best results have been obtained when packing under ethanol. The fillings produced from these alloys are also very prone to dramatic corrosion. The jury is out over the long-term toxicological effects of gallium, which has a clean record so far.
Experimental silver-coated intermetallic particles have been cold welded under pressure to fill well-supported cavities. Unfortunately, these are not the ones in which amalgam is the most useful.
Resin-Based Composite Fillings
These tooth-coloured filling materials have reached a high degree of sophistication since their appearance on the dental scene in the early 1960s. A modern dental composite consists of a paste created by combining a mixture of dimethacrylate monomers and cross-linking agents (known in dentistry as resins) with up to 80% by weight of silane-coated, ceramic particles (the filler), whose sizes range from 0.04-4 microns. This composite paste is packed into a dental cavity and the dentist exposes it for about 30 seconds to intense visible blue light. The light activates a chemical initiator within the composite and the resins undergo free radical addition polymerisation via their vinyl groups, turning the paste into a durable, solid filling.
Disadvantages of Resin-Based Composite Fillings
Composite fillings have similar strengths to amalgam but they tend to wear away more rapidly. They also shrink as they polymerise, and efforts have to be made to prevent gaps forming between the composite and the tooth. Incremental packing and curing helps, but the dentist uses other techniques and other materials to help form a seal.
Enhancing the Bond between Resins and Teeth
If dental enamel is present, its prismatic structure of apatite (calcium phosphate) can be etched with phosphoric acid to produce mini chasms, into which the resin matrix material of a composite will flow. When this sets, it results in strongly retentive mechanical bonds. However, materials scientists have spent many hours seeking to produce a bond to the dentine, which exists below the layer of protective, inorganic enamel.
Dentine is a wet, porous and sensitive combination of organic and inorganic materials, and current approaches to bond formation involve the use of primers containing bifunctional compounds (table 1). These have hydrophilic molecules at one end and hydrophobic ones at the other. The hydrophilic ends infiltrate the wet dentine and the hydrophobic ends form links with the resins in the composite, and so the composite is bonded to the dentine.
Table 1. Bifunctional primers are molecules with characteristic chemical groups at each end. These groups have an affinity for one particular sort of surface. In dentistry, they are used to form bonds between dissimilar groups.
|
|
|
Silane
|
Used to link silica-based porcelains and glass-ceramics to dental resins. They have resin-seeking groups connected to ceramic-seeking groups.
|
Resin
|
Ceramic
|
|
4-META and MDP
|
Used with resin-based dental cements to attach base-metal bridges to dental enamel. They have resin-seeking groups connected to metal-seeking groups.
|
Resin
|
Metal
|
|
Dentine bonding agents
|
These have hydrophobic groups (which bond to water hating resins) connected to hydrophilic groups (which allow them to infiltrate wet dentine). They are used to link dental resins to dentine.
|
Hydrophobic
|
Hydrophilic
|
4-META = 4-metacryloxyethyl trimellitate anhydride, MDP = 10-methacryloxydecyl dihydrogen phosphate.
Ion-Leachable Glass Cements
The first aesthetic, tooth-coloured filling materials appeared in the second decade of the 20th century. These were the silicate cements, which were formed when phosphoric acid displaced metal ions from a glass made from alumina, silica and several other metal oxides and fluorides. They set when aluminium phosphate was precipitated between the glass particles. These cements were used by dentists for half a century to fill cavities in front teeth, for not only did they match the colour and translucency of enamel and dentine, but they also acted as a source of fluoride. It was unusual to see dental decay recurring in any tooth they were used to fill.
Similar cements also form when variations on this type of glass are exposed to polymeric acids which possess carboxylate groups. The acids displace metallic ions from the glasses and these cross-link the polymeric acid chains causing the cement to set. The acids also undergo ion exchange reactions with the apatite (calcium phosphate) crystals, which form part of both dentine and dental enamel. These glass ionomer cements, as they are known, thus form direct chemical bonds to teeth, without the need for the primers described above.
However, the basic cements lack the strength and resistance to wear that the dental composites have, and recent research has come up with resin-modified versions. These possess not only the carboxylate groups needed to form bonds to teeth, but also the light-curable dimethacrylate components present in the composite resins. Their durability is thus considerably enhanced.
Cast Metal Restorations
For ninety years, dentists have been replacing the damaged crowns of molar teeth with gold alloys. These have been cast by the lost-wax process. In this process, a wax crown is invested in a wet silica-gypsum mixture. Once this has hardened, the wax is burned away and molten gold-copper-silver-palladium-platinum-zinc alloy is cast under pressure into the space left behind. Some of the alloys can be heat treated to form super-lattices and increase their strength. This makes them suitable for the construction of dental bridges, which replace a missing tooth (or teeth) either by cantilevering an artificial tooth from an adjacent tooth, or by suspending it (or them) between two such teeth. In either case the supporting teeth will have been cut down to accommodate a close fitting casting, which is cemented into place.
Bonded Restorations
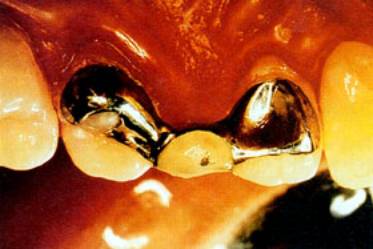
Since the 1960s, alloy-porcelain combinations, known to the dentist as bonded restorations have been available. These porcelain-covered metal castings combine the strength of a metallic superstructure with the aesthetic appearance of dental porcelain, creating the illusion that the restorations are real teeth. Alloys have been developed to which dental porcelains form durable retentive bonds, and many of these are now based on nickel-chromium. These metal frameworks are so rigid that they can be bonded via composites to the backs of acid etched teeth, thus eliminating the need for cutting down sound teeth, figure 1. Just as etching dental enamel creates retentive ‘chasms’, these nickel-chromium alloys can be electrolytically etched to produce features that allow the formation of mechanical bonds with resin-based composite cements.
|
|
|
Figure 1. Internal view of a dental bridge bonded via a resin-based cement to the backs of acid-etched teeth.
|
The oxides that form on these alloys can also be used to promote chemical links to cements via bifunctional primers, thus eliminating the challenge of producing a uniformly etched surface.
Dental Ceramics
Ceramic materials have the ability to emulate natural teeth, and they are some of the oldest dental materials, going back to 1792, when complete dentures were made from them. In 1996 they are used to create inlays, veneers, and crowns, as facings on metal substrates, and even as bridges, which can be made completely from high-strength ceramics. Restorations in ceramics are generally made by building up the correct aesthetic combinations of prefired, pigmented particles, and then re-firing under vacuum to sinter them together and eliminate voids.
Overcoming the Brittle Nature of Ceramics
The developers of modern dental ceramics, aware of their inherent brittle nature, have discovered many ways of interfering with the propagation of cracks within them. To this end, dispersion strengthening with alumina was the first approach. However, because of the opaque nature of the alumina, it is limited to the inner most structure of a crown, known to the dentist as a core.
The cracks, which lead to catastrophic failure, nucleate at the internal interface between the prepared tooth and the ceramic crown. A high strength core can prevent the growth of these cracks and the strongest cores are currently made from either alumina or zirconia. The toughest all-ceramic core produced so far actually infills any cracks in a high alumina base with molten glass during a firing stage in its production.
Other approaches to crack inhibition have included low temperature ionic crowding, a process in which small atoms (such as sodium) present in the surface of porcelain are exchanged for larger ones (such as potassium) by immersing the solid material in fused potassium nitrate. This produces a compressive stress in the surface, which constrains the opening of any cracks. Cast glass-ceramics have also appeared, and these are given post-casting heat treatments that produce reinforcing mica-like crystals within the glass.
To bond brittle porcelain to a strong and rigid metal substrate, special porcelaind have been developed with thermal expansion characteristics that match those of the metal. This in turn prevents high interfacial stresses being created between the two as they cool.
Glass Ceramics
Glass ceramics are also used in several CAD/CAM applications in dentistry. In one of these a restoration is designed on a video image of a prepared tooth. It is then machined from a pre-fired block of glass ceramic. All of this takes place in front of the patient. As with all types of ceramic restoration, the machined unit is then coated with a silane bonding agent and cemented to the tooth with a resin-based cement. The tooth itself is also coated with an enamel/dentine bonding agent.
Dental Implants
For years people have been under the impression that the dentist was able to ‘screw in’ teeth to replace those which were missing. However, what they had experienced was the use of one type of metal post. Posts can be either cemented or screwed into the canals of teeth that have lost their crowns but still have their roots. Such teeth are ‘root-treated’ to remove their nerves and blood supplies, and onto the posts ceramic or ceramic metal crowns are themselves cemented.
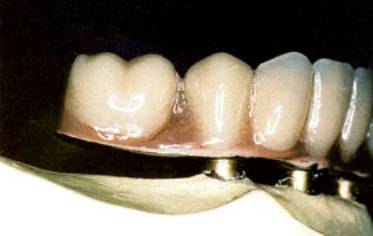
Although many attempts have been made to replace missing roots with all sorts on metallic implants, the satisfactory use of a screwed in implant goes back only to the mid 1980s. In practice the gum is slit, and a hole is cut slowly in the bone and then tapped under a continuous flow of sterile, cold water to prevent it being damaged by over-heating. A cold-worked Grade 4 commercially pure titanium screw is then inserted slowly and covered with gum tissue for 6 months. During this time the bone grows into intimate contact with the passive oxide layer on the titanium and it is said to be osseointegrated. The gum tissue is cut once more and a titanium sleeve is screwed onto the implant. This will ultimately pass through the healed gum. Onto these sleeves a metallic superstructure can be screwed and this can support, for example, a polymeric denture base and artificial teeth, figure 2.
|
|
|
Figure 2. Side view of a superplastically-formed, titanium alloy, cantilevered superstructure, attached to dental plaster analogues in a plaster model of a patients jaw.
|
For many years these superstructures have been cast in gold alloys and getting them to sit perfectly on the titanium sleeves has been a challenge of the highest order. However, titanium frameworks are currently being investigated, particularly those constructed by alternative routes to casting, and considerable promise is being shown by those made by superplastic forming. This is undertaken in an inert atmosphere at 900°C on reinforced refractory models.
Ceramics have also been tried as dental implants. However, because of their brittleness and the smallness of the structures, their optimal role has been as coatings on metal implants. Titanium implants, for example, have had their surfaces coated with hydroxyapatite to try and help osseointegration, and surface active glasses (‘bioglasses’) have been used for the same purpose. Also, by placing them in the holes left behind after the extraction of teeth, these glasses have shown promise in preventing bone resorption.
|