Updated by Reginald Davey 23/11/22
Mercury (II) iodide is a chemical compound that exists in red-orange crystals. It is highly reactive to lead salts, iodoform, chlorides, light, heat, hydrogen peroxides, and copper salts. Unlike mercury (II) chloride, it is hardly soluble in water. When heated above 126°C, it undergoes a phase transition from the red alpha crystalline form to a pale yellow beta form and exhibits thermochromism.
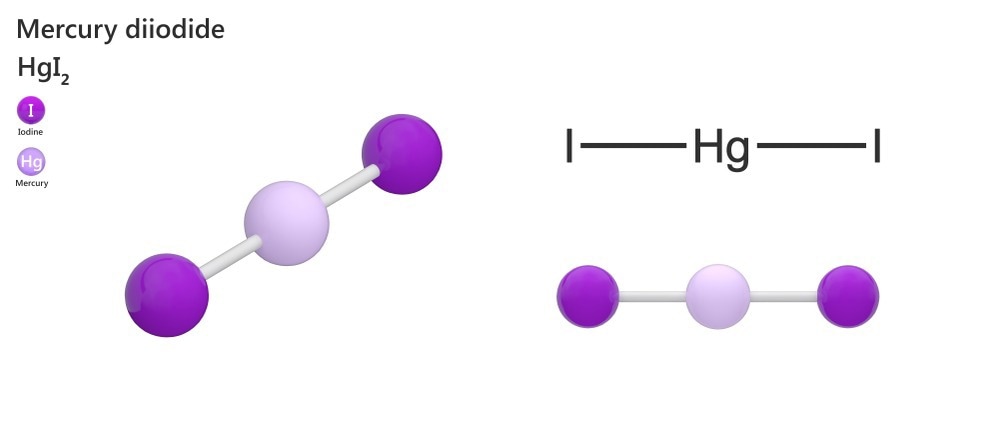
Image Credit: Orange Deer studio/Shutterstock.com
A third, orange colored-form of mercury (II) oxide is formed during recrystallization. This form is metastable and eventually converts back to the red form. Other names for mercury (II) iodide include mercury diiodide, diiodomercury, and mercuric iodide. The chemical formula of mercury (II) iodide is HgI2. Mercury iodide is light-sensitive and insoluble in an aqueous solution, sinking when added to water.
Aside from mercury (II) iodide, there are several mercury derivative compounds that are used in multiple industrial applications. These include mercury (I) chloride, mercury (II) selenide, mercury fulminate, mercury (II) sulfide, and mercury zinc telluride.
Manufacture
Mercury iodide is produced by adding potassium iodide in its aqueous form to aqueous mercury (II) chloride solution. This solution is stirred and then filtered to remove the precipitate and dried at 70oC to create commercial-grade mercury (II) iodide. It is also a common precipitate in many other chemical reactions.
Naturally-Occurring Mercury (II) Iodide
Whilst it is mainly produced via synthetic means, mercury (II) iodide can be found in nature, in the form of coccinite, an extremely rare mineral. This mineral was first discovered in Mexico, but deposits have been reported in New Zealand and Germany. It was initially reported by del Rio as lemon-colored spots in sandstone located at Casas Vijecas in Mexico.
Coccinite has a tetragonal crystalline structure, uniaxial optical properties, and a specific gravity of 3.17. It is translucent. Its average refractive index is 2.684, has a birefringence of 0.193, and is volatile at room temperature. Additionally, like laboratory-prepared mercuric iodide, coccinite is highly toxic.
Crystalline Structure
Mercury iodide can exist in many different crystalline structures. Due to this, the phase diagram of this compound is complex.
Applications
Mercury iodide has a number of key commercial and industrial applications. In veterinary medicine, it is routinely employed as a blister ointment to treat conditions such as bursal enlargements and exostoses. It is also commonly used to prepare Nessler’s reagent for the detection of ammonia.
This compound has found useful applications in semiconductor research in the past few years. Mercury itself is a well-recognized semiconducting material, with mercury (II) iodide commonly used in X-ray and gamma-ray detectors. It is also used in room-temperature imaging applications.
One of the key properties which makes mercury and its derivative compounds, such as mercury iodide, useful for semiconductor applications is its outstanding electrical conductivity. Furthermore, mercury compounds are high-density liquids in their room-temperature forms, which means that they save space within the semiconductor device.
Chemical Properties
The chemical properties of mercury (II) iodide are provided in the table below:
| Chemical Properties |
| Chemical Formula |
HgI2 |
| Molecular Weight |
454.4 |
| CAS No. |
7774-29-0 |
| Group |
Mercury – 12
Iodine- 17 |
| Crystal Structure |
Tetragonal |
Electrical Properties
The electrical properties of mercury (II) iodide are provided in the table below:
| Electrical Properties |
| Magnetic Susceptibility |
1.65x10-4 |
Thermal, Mechanical and Optical Properties
The thermal, mechanical and optical properties of mercury (II) iodide are provided in the tables below:
| Thermal Properties |
| Heat of Fusion |
84 J/g |
| Heat of Formation |
-105.4 kJ/mol |
| Standard Molar Entropy |
180 J/mol K |
| Mechanical Properties |
| Density |
6.36 g/cm3 |
| Melting Point |
259°C |
| Boiling Point |
354°C |
| Optical Properties |
| Refractive Index |
2.45 |
Safety Information
| Safety Information |
| GHS Hazard Statements |
H300 - Fatal if swallowed
H310- H310: Fatal in contact with skin
H330- Fatal if inhaled
H373-May cause damage to organs through prolonged or repeated exposure
H410 - Very toxic to aquatic life with long lasting effects. |
| Safety Precautions |
S13 - Keep away from food, drink and animal foodstuffs
S28 - After contact with skin, wash immediately with plenty of water
S45 - In case of accident or if you feel unwell seek medical advice immediately
S60- This material and its container must be disposed of as hazardous waste
S61- Avoid release to the environment. |
In Summary
Mercury (II) iodide is a mercury compound that possesses outstanding physical, electrical, thermal, and chemical properties which make it suitable for use in semiconductors, ointments, and ammonia detection via the preparation of Nessler’s compound.
However, like all mercury derivatives, mercury iodide is extremely toxic to humans and the environment, requiring strict handling and disposal methods in laboratory, medical, and industrial environments.
More from AZoM: What are Polyoxometalate Soft Matter Composites?
Further Reading and More Information
Mindat (website) Coccinite [online] mindat.org. Available at:
https://www.mindat.org/min-1100.html
Hostettler, M & Schwarzenbach, D (2005) Phase diagrams and structures of HgX2 (X = I, Br, Cl, F) Comtes Rendus Chimie 8:2 pp. 147-156 [online] sciencedirect.com. Available at:
https://www.sciencedirect.com/science/article/pii/S163107480400336