The researchers also demonstrated the use of alginate and its metal chelating properties. The copper-doped photocatalysts improved the mechanical and photocatalytic properties and provided excellent resistance to attrition and sample handling compared to powder catalysts.
Why use Nanostructured Photocatalysts in Advanced Oxidation Processes?
The main technique used in water treatment is Advanced Oxidation Processes (AOPs). This technique involves the generation of highly reactive species such as hydroxyl radical (OH•), superoxide anion radical (O2−•), hydrogen peroxide (H2O2), oxygen(O2), and ozone for the treatment of water. The use of nanostructured photocatalysts provided certain advantages like high thermal stability, biological and chemical inertness, low cost and toxicity, mineralizing of water and CO2.
The use of powder catalysts posed disadvantages such as time and energy-consuming separation/recovery of the photocatalyst from the treated solution, mass transfer limitations, and the insufficient irradiation of the photocatalysts in the slurry emanating from shielding effects of the suspended solid. This leads to a significant increase in the operational and capital costs due to the use of high-power UV irradiation sources and stirring equipment.
About the Study
The researchers synthesized polymerized nanostructured beads by preparing a dope solution, and the TiO2 nanoparticles were dispersed and stabilized in alginate—a metal-binding polymer. Due to the metal-chelating properties of alginate, it was cross-linked with copper.
To obtain the precursor molds, two different coagulative agents (copper solution and glutaraldehyde) were considered. The precursor molds were obtained and the ceramic beads were synthesized by calcination/sintering in the air or by pyrolysis/sintering to prevent the beads from potential degradation due to continuous UV radiation, the oxidative action of the photocatalyst, and deterioration of the mechanical stability of the material.
The morphological and structural properties of the beads were analyzed by different techniques such as N2 porosimetry, scanning electron microscope (SEM), and X-ray diffraction (XRD). The adsorption of an azo dye like methyl orange was further studied in dark under UV radiation via batch experiments.
The samples were assigned different nomenclatures such as B1_600 (copper crosslinking), B1_600_air (copper crosslinking with air drying), and B2_600 (glutaraldehyde cross-linking).
Observations
Two cross-linking techniques were involved in the synthesis of the precursor Cu-alginate/TiO2 molds and two thermal procedures like calcination and pyrolysis were employed for the synthesis of ceramic photocatalytic beads.
The SEM results revealed details about the external surfaces of the sample. The direct use of Cu2+ as the cross-linking agent revealed the formation of densely structured, large, and elongated aggregates of TiO2 nanoparticles of size 2-3 μm and the interaggregate space of 1-2 μm. Whereas, the use of glutaraldehyde as cross-linking agent revealed that the aggregates were smaller in size and did not exceed the size of 1 μm and the interaggregate space was about 500 nm.
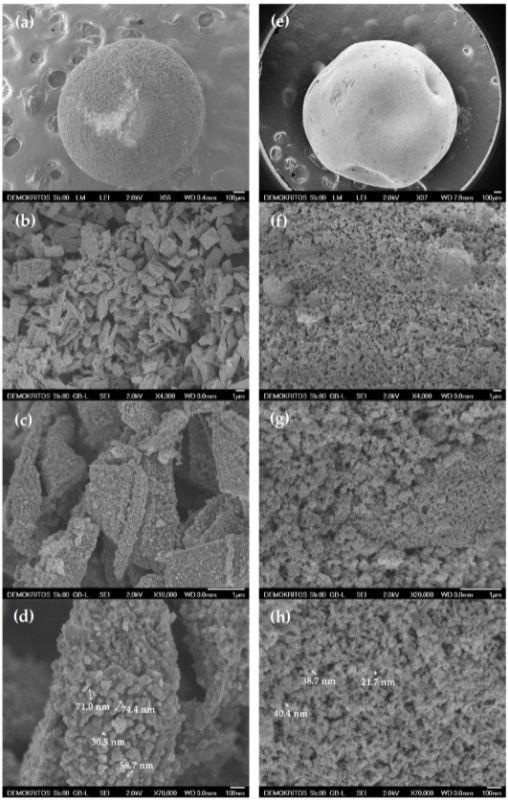
SEM images of the external surface of (a–d) B1_600 and (e–h) B2_600 samples. Image Credit: Theodorakopoulos, G.V et al., Materials
The LN2 porosimetry experiment was performed to study the textural and structural properties. The total pore volumes (TPV) and Brunauer, Emmett, and Teller (BET) surface area were analyzed. B1_600 exhibits lower TPV and surface area compared to B2_600 and larger pores were observed in the inter-aggregate space in B1_600 because of the presence of residual carbon and metal nanoparticles formed during pyrolysis.
The adsorption of MO dye and photocatalytic activity of beads exhibited high efficiency due to the presence of adsorbents like activated carbon present in the photocatalytic pores. The photocatalytic activity of three types of beads was evaluated in a 12 ppm MO solution.
The B2_600 samples with high TPV showed the highest adsorption capacity of 3.0 mg/g, and this was attributed to the presence of residual carbon. Another reason for high adsorption was the interaction mechanism driven by the π-π dispersion interaction between the adsorbate and the residual carbonaceous phase or the electron donor-acceptor complex mechanism. The B2_600 samples showed the least adsorption capacity of 0.1 mg/g.
The photocatalytic dye degradation is due to pyrolytic treatment along with the incorporation of copper species with TiO2. The pore volume and the surface properties also promote the production of active MO adsorbent sites.
The increase in the photocatalytic efficiency is due to adsorption on the photocatalytic surface which directly increases the pollutant’s local concentration, and also due to electron transfer between the semiconductor and metal nanoparticles which in turn inhibits the electron-hole recombination. The copper nanoparticles serve as a sink for TiO2 derived electrons during irradiation.
Conclusion
To conclude, the photocatalytic ceramic nanobeads showed significantly enhanced mechanical properties, resistance to attrition, and high photocatalytic efficiency due to the doping with carbon nanoparticles. Photocatalysis showed more efficient results compared to powder catalysis. Residual carbon produced during pyrolysis increased the adsorption capacity because of the porous structure of carbon.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Theodorakopoulos, G.V.; Katsaros, F.K.; Papageorgiou, S.K.; Beazi-Katsioti, M.; Romanos, G.E. Engineering Commercial TiO2 powder into Tailored Beads for Efficient Water Purification. Materials 2022, 15, 326. https://www.mdpi.com/1996-1944/15/1/326