May 29 2008
Amyloid deposits in tissues and organs are linked to a number of diseases, including Alzheimer’s, Parkinson’s, type II diabetes, and prion diseases such as BSE. However, amyloids are not just pathological substances; they have potential as a nanomaterials. “The potential applications of these supramolecular assemblies exceed those of synthetic polymers,” state Ehud Gazit and co-author Izhack Cherny in the journal Angewandte Chemie from Wiley, “since the building blocks may introduce biological function in addition to mechanical properties.”
Even in nature, amyloids are not merely abnormal, incorrectly folded proteins; they are physiological components of organisms. For example, they are an important protective material in the egg envelopes of insects and fish. They are also involved in the formation of the biofilms of many bacteria, a coating on the surface of the bacterial cells that protects them from antimicrobial substances and facilitates their attachment to surfaces.
Amyloid fibrils are bundles of highly ordered protein filaments made of ladder-like strands and can be several micrometers long. In cross-section, amyloids appear as hollow cylinders or ribbons. Although amyloid fibrils are proteins, they more closely resemble synthetic polymers (plastics) than the usual globular proteins. Amyloids can display amazing mechanical properties similar to spider silk. Spider silk is, by weight, significantly stronger than steel and can be stretched to many times its original length without tearing— properties that have not been reproducible with synthetic fibers.
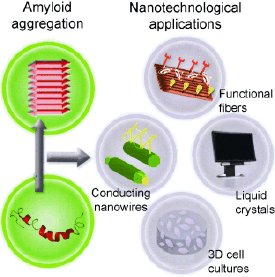
“The self-assembly properties of amyloids, together with their observed plasticity, makes them attractive natural building blocks for the design of new nanostructures and nanomaterials,” according to the authors from the University of Tel Aviv (Israel). “These building blocks can be broadly varied by means of simple molecular biological techniques.” Surfaces could be given tailored and biocompatible coatings, for example, in analytical flow devices for medical technology or bioanalysis. Other ideas include amyloid hydrogels for the encapsulation and controlled release of drugs and for scaffolds for three-dimensional cell cultures and tissue engineering. Functional proteins such as enzymes could be bound to amyloid-forming sequences to mimic biological processes.
Amyloid fibrils are also suitable as matrices for nanostructures. For example, it has been possible to produce a conducting nanoscale coaxial cable by filling amyloid nanotubes with sliver and externally coating them with gold.