Teknor Apex Company has separated production of medical-grade PVC compounds containing DEHP and other phthalate plasticizers from that of compounds plasticized with non-phthalate alternatives, the company's Vinyl Division announced today at MD&M West (Booth 2033).
The changeover has taken place at two Teknor Apex compounding plants in the United States and could be carried out at the company's facility in Singapore if demand warrants, according to industry manager Peter M. Galland. In addition to supplying medical-grade vinyl compounds worldwide, Teknor Apex also produces plasticizers for captive use and for sale on the merchant market, including phthalates and all important alternative types (see table).
"While DEHP has been used in medical devices for half a century with no recorded adverse human health interactions, regulatory initiatives like the European Union labeling requirement for DEHP, effective in 2010, compel some device manufacturers to look for compounds containing alternative plasticizers," said Galland. "Teknor Apex is well prepared to supply medical compounds regardless of plasticizer type, including DEHP, other phthalates, and the full range of non-phthalates."
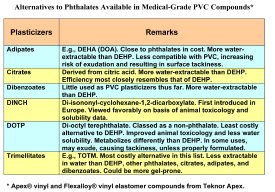
DEHP is by far the most widely used plasticizer in medical-grade vinyl and the least expensive. The most well-studied plasticizers after DEHP, in terms of animal toxicology and other testing, are other phthalates, such as di-n-octyl phthalate, DIOP, DINP, DPHP, and DIDP. Compounds containing these are roughly 3 to 7% more expensive than DEHP-plasticized vinyl. Three of them—DINP, DPHP, and DIDP—are less water-extractable than DEHP and thus less prone to be absorbed into the bloodstream and contacted tissues.
Compounds containing non-phthalate plasticizers range from 10 to 35% more expensive
than DEHP, with the exception of one, di-octyl terephthalate or DOTP, that is actually priced at a slight to nominal premium above DEHP. (In spite of its chemical name, DOTP is classed as a non-phthalate.). Besides higher cost, most non-phthalates pose performance tradeoffs in comparison with DEHP, particularly in regard to water extractability and tendency to exude, which can lead to surface tackiness. In addition, all of the non-phthalates have had limited use histories; hence human toxicology data is limited or lacking.
Even greater tradeoffs, however, are involved in switching from PVC altogether, according to Galland. "The medical device industry has repeatedly been exposed to 'PVC-free' medical tubing alternatives that kink, neck down when stretched, and resist solvent cementing at connections," Galland said. "These physical failings put patients at real risk."