May 5 2015
Researchers from Rice University have been analyzing the characteristics of a 2D dichalcogenide - molybdenum disulfide - and discovered that its atomically thin layers could adopt the characteristics of plastic in the presence of a sulfur-infused gas at a specific pressure and temperature.
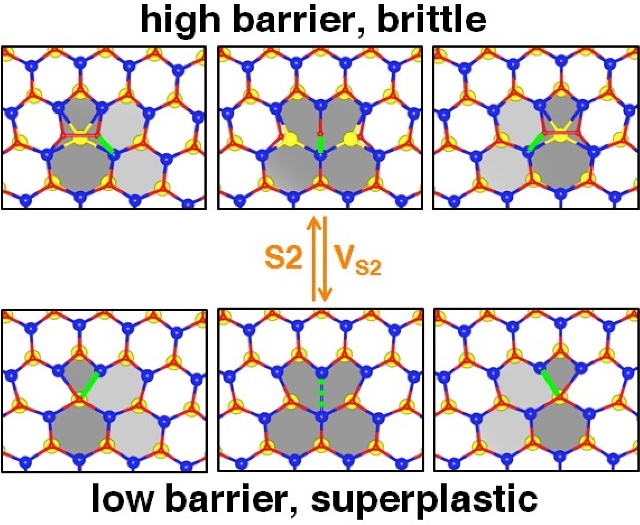 Calculations by Rice University scientists show that a two-dimensional layer of molybdenum disulfide can become superplastic by changing its environmental conditions. In an atmosphere with sulfur and under the right temperature and pressure, the energy barrier is lowered, allowing dislocations along the grain boundaries to shift and changing the material’s properties. S2 refers to a disulfur molecule; VS2 is a two-sulfur-atom vacancy. (Credit: Xiaolong Zou/Rice University)
Calculations by Rice University scientists show that a two-dimensional layer of molybdenum disulfide can become superplastic by changing its environmental conditions. In an atmosphere with sulfur and under the right temperature and pressure, the energy barrier is lowered, allowing dislocations along the grain boundaries to shift and changing the material’s properties. S2 refers to a disulfur molecule; VS2 is a two-sulfur-atom vacancy. (Credit: Xiaolong Zou/Rice University)
The Rice research team was led by Boris Yakobson, theoretical physicist, and Xiaolong Zou, postdoctoral researcher. Their finding showed that molybdenum disulfide could be deformed without necessarily breaking it. This could be of immense interest to materials scientists working on 2D materials. The study has been published in the American Chemical Society journal Nano Letters.
The key reason for choosing molybdenum disulfide, an already well-researched material thanks to its semiconducting properties, for this study was the interesting structure of its grain boundaries. While 2D materials such as graphene are flat sheets, 2D molybdenum disulfide has molybdenum atoms sandwiched between two sulfur layers.
During growth in a furnace, two sheets tend to join at different angles, and the atoms located at the perimeter are forced to form “defective” arrangements at the juncture where they meet. These arrangements are termed as dislocations. The researchers explained that by controlling the gas medium environmentally, it could be possible to boost the movement of these arrangements. This in turn could provide superplasticity to the material, thereby enabling deformation of the material well past its typical breaking point.
Yakobson observed that though metal pipes can be bent, with further environmental changes the material becomes brittle again.
Generally, the coupling of chemistry and mechanics is quite rare and scientifically difficult to understand. Corrosion is the best example of how chemistry affects mechanical behavior, and the science of corrosion is still in development.
Yakobson
The Rice team pinpointed two mechanisms for molybdenum disulfide by which activation energy barriers can be surpassed and superplasticity can be attained. In the first mechanism, only a single atom of molybdenum in a dislocation moves under the influence of external forces. This is called direct rebonding. The second mechanism is known as bond rotation, where multiple atoms move in opposite directions. The researchers observed that direct rebonding had much lower barrier than that of bond rotation.
Through the rebonding path, the mobility of this defect changes by several orders of magnitude. We know from the mechanics of materials that brittle or ductile qualities are defined by the mobility of these dislocations. What we show is that we can affect the tangible property, the stretchability, of the material.
Yakobson
Yakobson stated that the plasticity of dichalcogenides could be further tuned, and that the deformations can be removed from a 2D dichalcogenide sheet by tweaking the dislocations “to allow them to rapidly diffuse away and vanish or to form interesting aggregated states.”
This would then enable simpler methods for dichalcogenides manufacture with specific mechanical or electrical properties to suit different applications.
We think of these two-dimensional materials as an open canvas, theoretically speaking. You can very quickly read and write changes to them. Bulk materials don’t have this openness, but here, every atom is in immediate proximity to the environment.
Rice graduate student Mingjie Liu and Zhiming Shi, an exchange graduate student in Yakobson’s group from Jilin University, Changchun, China, are co-authors of the paper. Yakobson is Rice’s Karl F. Hasselmann Professor of Materials Science and NanoEngineering, a professor of chemistry and a member of Rice’s Richard E. Smalley Institute for Nanoscale Science and Technology.
A multidisciplinary university research initiative grant through the United States Army Research Office and the Robert Welch Foundation supported the research. The researchers utilized the National Science Foundation-supported DAVinCI supercomputer administered by Rice’s Ken Kennedy Institute for Information Technology.
References