Mar 3 2016
A team of researchers from TU Graz partnered with the Wetsus research centre in The Netherlands to create electrically charged water using a floating water bridge. The “water bridge” phenomenon was first discovered in the 19th century, but it was forgotten until it's scientific rediscovery in 2007 at TU Graz.
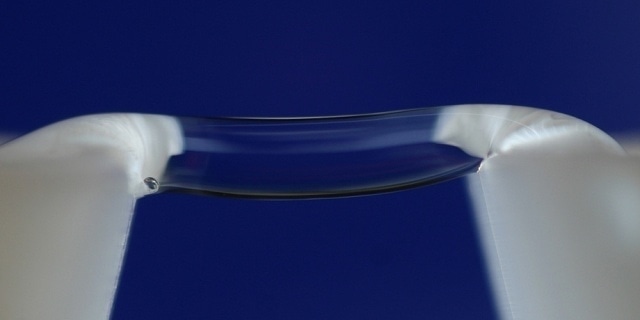 The water bridge pictured is formed under the influence of a high-voltage electrical field of about 15kV. It spans about 1 cm across two Teflon beakers, each filled with deionizer water. © Woisetschläger/Fuchs - TU Graz.
The water bridge pictured is formed under the influence of a high-voltage electrical field of about 15kV. It spans about 1 cm across two Teflon beakers, each filled with deionizer water. © Woisetschläger/Fuchs - TU Graz.
To illustrate this phenomenon, if very pure water that has been distilled several times is transferred into two beakers and exposed to a high voltage, the water begins to move up the side of each beaker forming a floating water bridge between the two beakers. The water across this bridge flows in both directions, and possesses a totally new state, with its own unique properties of structure and density.
The collaborative team explained that this floating water bridge generates electrically charged water and stores the charge for a short period of time.
Protonic electrical charge
The water is not charged electronically, but protonically. This unique water is either negatively or positively charged, based on whether it possesses fewer or more protons. The research reveals that in anodic water, which is water with a positive charge, protons are generated in relation to the occurring electrolysis.
These hydrogen nuclei flow from the first beaker, via the water bridge, into the cathodic water in the second beaker, which contains a negative charge. In the second beaker the hydrogen nuclei are neutralized by hydroxyl ions. A surplus of protons can be found in one water beaker, while in the other beaker there are no protons. This is because the protons move at a limited speed. If the water bridge is abruptly turned off, the proton charges still remain. This can be measured using impedance spectroscopy. The initial experiments reveal that the charge in the water remains stable for a week.
From "water battery" to low-waste chemistry
This breakthrough concept of using water bridges in biochemical or electrochemical reactors paves the way for a number of probable industrial applications. Using the water bridge, substances can be made to come into contact with other materials to facilitate chemical reactions, water can be transformed into a “water battery” to store electric charge, and acids and alkalis can be created without the need for any opposing ions - with no acid and alkaline water. All of these applications open up further possibilities for minimal waste from chemical processes, eco-friendly cleaning agents, and new opportunities for medical applications.