Apr 22 2016
More than four billion tons of uranium exist in the oceans. This huge quantity would be sufficient to meet the global energy requirements for the next 10,000 years, only if the element could be captured from seawater to fuel nuclear power plants.
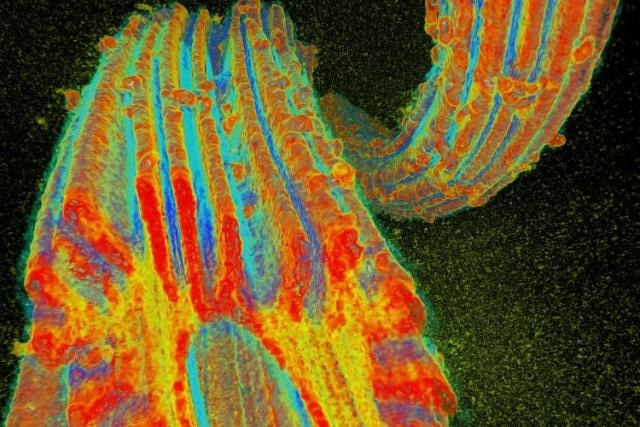 Oak Ridge National Laboratory researchers developed a fiber to adsorb uranium from seawater. Researchers at Pacific Northwest National Laboratory exposed the fibers to Pseudomonas fluorescens and used the Advanced Photon Source at Argonne National Laboratory to create a 3-D X-ray microtomograph to determine that the fiber structure was not damaged by the organism. (Image credit: Pacific Northwest National Laboratory, U.S. Dept. of Energy)
Oak Ridge National Laboratory researchers developed a fiber to adsorb uranium from seawater. Researchers at Pacific Northwest National Laboratory exposed the fibers to Pseudomonas fluorescens and used the Advanced Photon Source at Argonne National Laboratory to create a 3-D X-ray microtomograph to determine that the fiber structure was not damaged by the organism. (Image credit: Pacific Northwest National Laboratory, U.S. Dept. of Energy)
A report on the significant improvements in this area has been published in the Industrial & Engineering Chemistry Research by the American Chemical Society’s (ACS).
For half a century, researchers from all over the world had limited success when attempting to mine uranium from the oceans. In the 1990s, scientists from Japan Atomic Energy Agency (JAEA) pioneered materials with uranium stuck to them or materials that were immersed in water with uranium absorbed onto their surfaces. In 2011, the U.S. Department of Energy (DOE) started a program with the help of a multidisciplinary team from U.S. research institutes, universities and national laboratories to present a talk on the basic economic challenges of extracting uranium from seawater. Within a period of five years, the team produced new adsorbents that help to decrease the uranium extraction cost by three to four times.
To document this and a number of other successes, the special issue concentrated on “Uranium in Seawater” amasses research delivered by international scientists at the spring 2015 ACS meet in Denver. Contributions came in from researchers, supported by DOE’s Office of Nuclear Energy’s Fuel Resources Program. This program is a global initiative, involving researchers in Japan and China under agreements with the JAEA and Chinese Academy of Sciences. The DOE program sets the technological foundation to establish the economic feasibility of recovering uranium from seawater. It assists researchers at national research institutes, universities, and laboratories focused on producing and testing the future generation of adsorbents that will have the potential to display faster binding, higher adsorbent capacity, and lower degradation over several use cycles in seawater.
For nuclear power to remain a sustainable energy source, an economically viable and secure source of nuclear fuel must be available. This special journal issue captures the dramatic successes that have been made by researchers across the world to make the oceans live up to their vast promise for a secure energy future.
Phillip Britt, DOE Program
Scientists from the Pacific Northwest National Laboratory (PNNL) in Washington and Oak Ridge National Laboratory (ORNL) in Tennessee - two DOE labs - headed more than half of the 30 papers published in the special issue. ORNL contributions focused on synthesizing and categorizing uranium adsorbents, and the PNNL papers concentrated on marine testing of adsorbents synthesized at universities and national labs.
Synthesizing a material that’s superior at adsorbing uranium from seawater required a multi-disciplinary, multi-institutional team including chemists, computational scientists, chemical engineers, marine scientists and economists. Computational studies provided insight into chemical groups that selectively bind uranium. Thermodynamic studies provided insight into the chemistry of uranium and relevant chemical species in seawater. Kinetic studies uncovered factors that control how fast uranium in seawater binds to the adsorbent. Understanding adsorbent properties in the laboratory is key for us to develop more economical adsorbents and prepare them to grab as much uranium as possible.
Sheng Dai, Uranium from Seawater Program, ORNL
The teamwork led to the formation of polyethylene fiber braids containing amidoxime, which is a chemical species that attracts uranium. Until now, testing has been performed in the laboratory using real seawater, but the polyethylene fiber braids are capable of being deployed in oceans where nature will perform the task of blending, preventing the expense of pumping huge quantities of seawater through the fibers. After a number of weeks, uranium oxide–laden fibers are collected and then passed through an acidic treatment that desorbs or releases uranyl ions, regenerating the adsorbent for reuse. Continuous processing and improvment of the uranium resulted in developing a material capable of fueling nuclear power plants.
PNNL researchers examined the adsorbents produced at ORNL and several other laboratories, including universities taking part in the Nuclear Energy University Program, using both natural filtered and unfiltered seawater obtained from Sequim Bay in Washington, under monitored temperature and flow-rate conditions. Gary Gill, deputy director of PNNL’s Coastal Sciences Division, organized three marine testing site; the University of Miami in Florida, Woods Hole Oceanographic Institution in Massachusetts, and PNNL’s Marine Sciences Laboratory in Sequim, Washington.
Understanding how the adsorbents perform under natural seawater conditions is critical to reliably assessing how well the uranium adsorbent materials work. In addition to marine testing, we assessed how well the adsorbent attracted uranium versus other elements, adsorbent durability, whether buildup of marine organisms might impact adsorbent capacity, and we demonstrated that most of the adsorbent materials are not toxic. PNNL also performed experiments to optimize release of uranium from the adsorbents and adsorbent re-use using acid and bicarbonate solutions.
Gary Gill, Deputy Director, Coastal Sciences Division, PNNL
Marine testing at PNNL highlighted that an ORNL adsorbent material was capable of holding 5.2 grams of uranium per kilogram of adsorbent, after being exposed to natural seawater for 49 days. The Uranium from Seawater program still continues to produce major enhancements, and also helps to form adsorbents with increasing capacities to collect uranium. Recent testing exceeded 6 grams of uranium per kilogram of adsorbent after a period of 56 days in natural seawater. This is an adsorbent capacity 15% greater than the results featured in the special edition.
The special issue captures a variety of enterprises, including
-
Uranium coordination and computer-aided ligand design (ORNL)
-
Thermodynamic, kinetic and structural characterization of the adsorbent (Lawrence Berkeley National Laboratory, ORNL, PNNL)
-
Adsorbent synthesis using radiation to graft more polymer onto the polyethylene (ORNL, Brookhaven National Laboratory, University of Maryland)
-
Adsorbent synthesis using a chemical method (ORNL, University of Tennessee)
-
Adsorbent nanosynthesis (ORNL, PNNL, Hunter College, University of Chicago, University of South Florida, SLAC National Accelerator Laboratory, University of California–Berkeley)
-
Laboratory testing and modeling of adsorbent performance (ORNL, Georgia Tech)
-
Marine testing and performance assessment of the adsorbent (PNNL, Woods Hole Oceanographic Institution, University of Miami)
-
Adsorbent durability and reusability (PNNL, University of Idaho)
-
Adsorbent characterization, toxicity and biofouling studies (ORNL, PNNL, UI)
-
Technology cost analyses and modeling (University of Texas–Austin)
-
Green chemistry: Adsorbents prepared using marine shellfish waste (University of Alabama)
-
Adsorbent deployment (PNNL, ORNL, MIT)
Erich Schneider, from the University of Texas–Austin, pointed out that the uranium obtained from terrestrial sources has the potential to last for about 100 years. A depletion of terrestrial uranium may result in an increase in prices.
If we have technology to capture uranium from seawater, we can ensure that an essentially unlimited supply of the element becomes available if uranium prices go up in the future.
Erich Schneider, University of Texas-Austin
In July 2016, experts in extracting uranium from seawater will congregate University of Maryland–College Park for the International Conference on Seawater Uranium Recovery. At this meet, the experts plan to analyze benefits of uranium from seawater to sustain the world’s lights.
UT-Battelle controls ORNL for DOE’s Office of Science. ORNL executes applied and fundamental research to provide transformative solutions to compelling issues in security and energy.
Battelle supervises PNNL for DOE’s Office of Science. Interdisciplinary teams at PNNL cater to America’s most critical issues in national security, the environment, and energy by bringing about improvements in applied and basic science.
The only largest supporter of basic research in the field of physical sciences in the United States is the Office of Science, which is working towards addressing some of the extremely critical challenges.