Apr 21 2017
 This image shows the detail of the coordination spheres of the bromide anion with Et3BuN+Br? (CREDIT - M. Fourmigue)
This image shows the detail of the coordination spheres of the bromide anion with Et3BuN+Br? (CREDIT - M. Fourmigue)
While an IUPAC description of hydrogen bonding was only introduced in 2011 after years of discussions in the scientific community, it did not take such a long time to arrive at a similar definition of halogen bonding, following a resurgence of this interaction in the literature which dates back to the early 1990s, Fourmigué, M. (2017). Acta Cryst. B73, 138-139
The halogen-bonding interaction is principally defined as an electrostatic interaction between a charge-depleted area, called an σ-hole, and a charge concentration (Lewis base) that a covalently bound halogen atom displays in the extension of this bond.
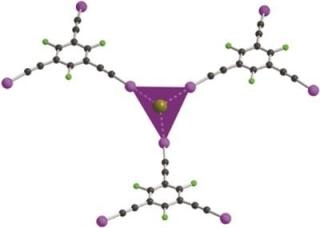
In the latest paper by Szell et al, (2017). Acta Cryst. B73, 153-162 single-crystal X-ray diffraction structures have been stated for a chain of seven halogen-bonded co-crystals featuring 1,3,5-tris(iodoethynyl)-2,4,6-trifluorobenzene as the halogen-bond donor, and bromide ions (as phosphonium or ammonium salts) as the halogen-bond acceptors. Based on the stoichiometry, the resulting frameworks can develop honeycomb structures of variable geometry, but also systems with six or four halogen bonds to the bromide ion. While the counter-cations normally inhabit the void spaces in the current work, the building of halogen-bonded frameworks with probable gas storage applications is an attractive prospect which may be simplified in the future by ligands enabling multidentate and directional interactions.