Oct 4 2017
The fact that solid glass acts similar to a very slow-moving liquid can be seen by looking at the waves at the bottom of old window panes. At present, an innovative research has confronted the concept that the atomic structure of glass is similar to that of a liquid, at least for a specific type of glass known as “amorphous ice” that is formed upon cooling water to extremely lower temperatures.
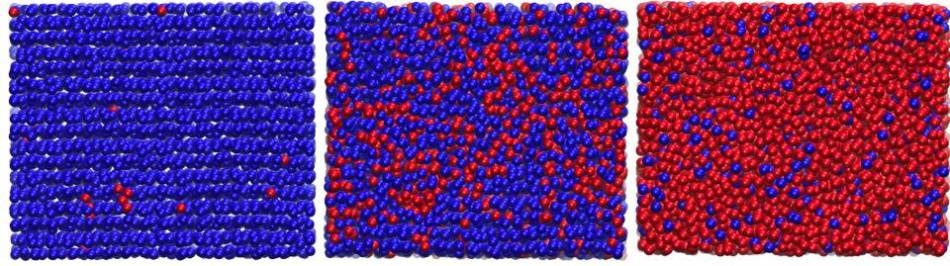 Shown above are representative snapshots of the compression of hexagonal ice to high-density amorphous ice. Blue and red spheres represent different local environments of water molecules based on their local order: blue for ordered, hexagonal ice-like environments and red for disordered, high-density amorphous-like environments. Left panel: sample of hexagonal ice at high pressure (before the phase transition). Middle panel: phase transition between hexagonal ice and high-density amorphous ice. Right panel: sample of high-density amorphous ice. CREDIT: Image courtesy of Fausto Martelli et al., Princeton University
Shown above are representative snapshots of the compression of hexagonal ice to high-density amorphous ice. Blue and red spheres represent different local environments of water molecules based on their local order: blue for ordered, hexagonal ice-like environments and red for disordered, high-density amorphous-like environments. Left panel: sample of hexagonal ice at high pressure (before the phase transition). Middle panel: phase transition between hexagonal ice and high-density amorphous ice. Right panel: sample of high-density amorphous ice. CREDIT: Image courtesy of Fausto Martelli et al., Princeton University
As part of the research, Scientists from the Princeton University and the City University of New York applied computer simulations to demonstrate that water molecules in amorphous ice are arrayed in a hitherto undiscovered order, which was not included in the original liquid. The discovery has been reported in the Physics Review Letters journal on September 29th, 2017 and can assist in elucidating the inquisitive and life-giving characteristics of water. It is also in disagreement with the interpretation of the term glass.
Glasses are originally formed by rapid cooling of a liquid. Based on prevalent knowledge, a glass takes over the arrangement that existed in the original liquid. However, regarding amorphous ice, when the liquid water gets cooled, a new and well-ordered arrangement of molecules takes place.
According to our results, these types of glass are not simply frozen liquids—this picture doesn’t hold anymore. We are essentially saying that a notion that scientists have believed for many years is partially wrong.
Fausto Martelli, Associate Research Scholar, the Department of Chemistry, Princeton University
Before this research, Scientists had knowledge of the fact that very fast freezing of water (which takes place at the exceptionally cold temperatures experienced in the outer space) culminates in the emergence of an unusual material different from normal ice. The material which is also known as amorphous ice does not have the extremely ordered crystalline structure similar to normal ice, causing Researchers to classify it as a glass—a liquid, the movement of which has slowed down to the speed of glaciers. Amorphous ices are very rare on Earth. However, universally, they are the most ample form of water.
The new research has discovered that the molecules in amorphous ices are ordered in a formerly unexplored internal pattern, called as disordered hyperuniformity. Disordered hyperuniformity is interpreted as an order over large spatial distances although there exists no order over short distances. Disordered hyperuniform materials can be classified between a crystal (extremely organized across long distances) and a liquid (only ordered across short distances).
“The existence of these large-scale structural correlations has not been fully appreciated, and that is really what we wanted to address in this study,” stated Salvatore Torquato, Co-author of the study and a Professor of Chemistry who worked in collaboration with Princeton Senior Scientist Frank Stillinger to be the first to identify hyperuniformity over 10 years ago. “The information present in these systems is quite striking, and leads to completely new insights about materials,” stated Stillinger.
From the time, he and his team have identified hyperuniformity in various cases, for example, arrangement of cells in the eyes of a chicken.
Apart from Martelli and Torquato, the Authors of the study were Princeton’s Ralph W. *31 Dornte Professor in Chemistry Roberto Car and Nicolas Giovambattista, an Associate Professor at Brooklyn College-The City University of New York. Torquato and Car collaborated with the Princeton Institute for the Science and Technology of Materials.
In order to analyze the internal structure of amorphous ices, Martelli applied a computer model that investigates the behavior of more than 8000 water molecules to imitate the consequences of cooling the water below 80 °K or -316 °F. At such low temperatures, the lack of heat in the water molecules renders them unable to move from one place to another, or rotate at the same place.
At temperatures equal to and less than this temperature, the Scientists noted that the hyperuniform arrangement was ascertained with the data derived using the computer simulation.
We are not used to looking for order on such large length-scales. However, mathematics allows us to shed light on patterns that our eyes are not able to see.
Fausto Martelli, Associate Research Scholar, the Department of Chemistry, Princeton University
It took a number of months to carry out the simulations on high-performance research computers, such as Princeton University’s TIGRESS clusters by means of the Princeton Institute for Computational Science and Engineering.
The simulation allowed the Scientists to question the characteristic of water, which has a number of anomalous behaviors rendering it distinctively suitable to support life. One of the anomalies is that ice in its crystalline form is less dense when compared to water in liquid form, thus enabling ice to float, in turn enabling life to prevail even under ice in oceans and lakes.
A probable interpretation of anomalies of water is that at extremely colder temperatures, water might exist in two liquid phases, as against only one liquid state that is known to us. Here, one liquid state is denser than the other state. Identifying the transformation of water between the high-density and low-density states has been difficult because of technical problems.
This research offers indirect assistance for the existence of both the states, at least through computer simulations. Giovambattista simulated the exertion of high pressure on the model and found out that application of pressure resulted in the transformation of the low-density state of amorphous ice into a high-density state. The transformation between the two states is congruent with the existence of water in two liquid forms.
Gaining in-depth knowledge of the long-range order existing in amorphous materials is an ongoing research path. This is because tapping hyperuniformity can result in more practical applications. The hyperuniformity of amorphous silicon might offer new ways to tune electronic characteristics. The potential to regulate the hyperuniform long-range order of a material might assist Researchers in developing high-strength ceramics or more durable glasses.
According to Martelli, amorphous ices can be synthesized in the lab, thereby establishing the proof of hyperuniformity.
The U.S. Department of Energy and the National Science Foundation funded this research through grants DE-SC0008626 and DMR-1714722, respectively.