Background
Goodfellow Ceramic & Glass Division are an award-winning company who supply specialist glass and ceramics for scientific and industrial use.
Goodfellow Ceramic & Glass Division aim is to understand your application and then supply the most appropriate materials or components to meet your needs.
Our technical staff are qualified in glass technology and materials science and can, therefore, provide impartial advice and full technical support for your projects.
Borosilicate Glass
Borosilicate glass, known under trade names such as Pyrex® and Duran®, is widely used in chemical and engineering applications.
Properties of Borosilicate Glass
Borosilicate glass is chemically resistant, has a low thermal expansion coefficient and can be used at relatively high temperatures. It is available in many forms and sizes such as rod, tube, plate and as machined or hot formed components.
Table 1. Typical properties of borosilicate glass.
| |
Property |
Units
|
Value
|
| General |
Density |
g/cm3
|
2.23
|
| Mechanical |
Young's Modulus |
GPa
|
64
|
| Thermal |
Max. Use Temperature |
°C
|
500
|
| Thermal Conductivity |
W/m.K
|
1.14
|
| Co-Efficient of Linear Expansion |
10-6/°C
|
3.3
|
| Electrical |
Volume Resistance |
Ù.cm
|
1015
|
| Dielectric Constant |
|
4.6
|
| Dielectric Strength |
kV/mm
|
30
|
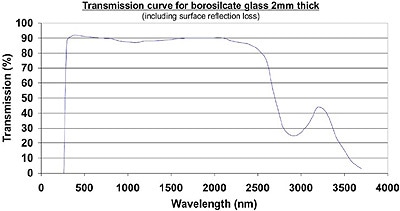
Figure 1. Transmission curve for 2mm thick borosilicate glass.
Properties of borosilicate glass shown are typical values, they are not absolute material properties, and should be used for guidance only. It is recommended that materials and components are tested for their suitability for a specific application.

This information has been sourced, reviewed and adapted from materials provided by Goodfellow Ceramic & Glass Division.
For more information on this source, please visit Goodfellow Ceramic & Glass Division