Nov 20 2017
A new catalyst, referring to a substance capable of activating oxidation processes in low-reactive components of gas and oil, has been developed by a team of scientists from the Research Institute of Chemistry (RIC) of RUDN University in collaboration with colleagues from major scientific centers.
The new method of hydrocarbon processing will help to efficiently develop valuable organic substances such as alcohols and acids, using a reaction that needs just minor heating without increased pressure. The results of the team's work have been published in the Journal of Organometallic Chemistry.
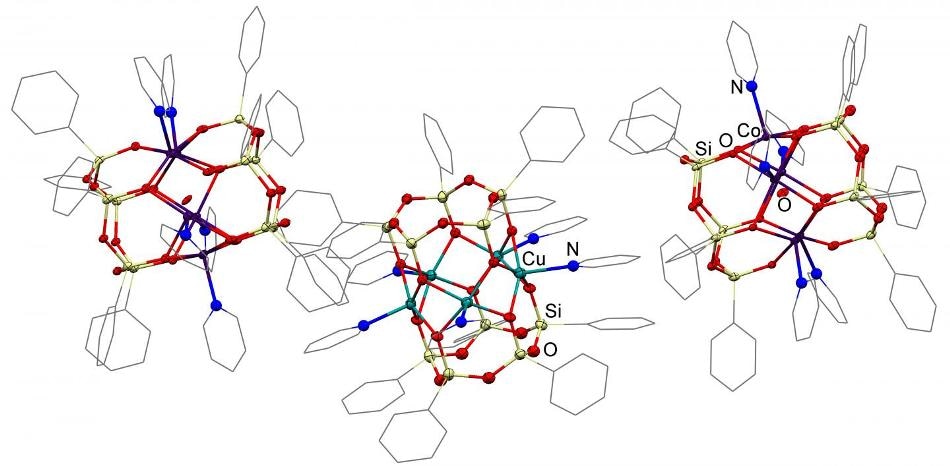 The structure of pentanuclear prismatic metallasilsesquioxanes (copper and cobalt-containing). CREDIT Alexey Bilyachenko
The structure of pentanuclear prismatic metallasilsesquioxanes (copper and cobalt-containing). CREDIT Alexey Bilyachenko
"The catalysts we've created contain silicon (or germanium) and metal (copper, iron, cobalt, etc.). They are able to easily break the bonds between carbon and hydrogen atoms both in saturated and unsaturated hydrocarbons (the main components of oil and gas) and turn them into valuable products: alcohols, acids, and ethers. This is a topical subject - some works on the activation of carbon-hydrogen bonds were shortlisted for the 2017 Nobel Prize in Chemistry," explains Alexey Bilyachenko, one of the co-authors of the work, candidate of chemical sciences, and deputy director of Research Institute of Chemistry RUDN University.
During their work, specialists applied synthetic methods employing the abilities of organic silicon and germanium derivatives in order to form unusual 3-dimensional structures incorporating atoms of varied metals. These framework compounds are considered to be soluble in organic solvents increasing the activity of a catalytical particle. Additionally, the structure of the matrix helps determining the direction of the "catalyst' attack" (for example, the oxidation of an organic molecule is targeted to specific positions of a reagent molecule).
The catalysts described in the work have been classified as prismatic metallasilsesquioxanes. These compounds are made up of a middle metal-containing layer placed between two layers of silicon-containing cycle. Every single atom of silicon is linked to an organic substitute.
Structural features and "nuclearity" of metal silsesquioxanes (that is, the number of metal atoms present in the compound) are extremely dependent on synthesis conditions, which cause specific difficulties for researchers. One of the key results of the team's work is to determine essential components of a reactive mixture that enables attaining a final product with a specific number of metal atoms, thus determining further properties of a catalyst.
The researchers specifically demonstrated the possibility of targeted production of pentanuclear products when synthesizing compounds containing cobalt, copper, and nickel ions with the use of a well-established heterocyclic compound pyridine. Remarkably, the products developed in other systems were hexanuclear.
Another vital discovery refers to the stability of the rare pentanuclear structure in the course of transition from solid matter to a solution. This was shown on the example of a copper-containing compound. After replacing pyridine with dimethyl formamide, a solvent known to be extensively used in lab work, the researchers discovered that both the target compounds and the source had the same pentanuclear structure by using XRD investigations. This signifies fairly high stability of the framework compound which is essential to extend the period of a catalyst's activity within a solution.
This work presents catalytical experiments which show that a pentanuclear copper-containing compound is efficient in homogeneous catalyst of the oxidation of secondary alcohol (to ketones) and alkanes (to alkylhydroperoxides) by using peroxides. Notably, these reactions occur in "mild" conditions, that is, without increased pressure and after minor heating. The discovered methods of gas and oil processing with the help of hydrocarbons activation with metal-containing compounds have a clear advantage over the usual pyrolysis and cracking technologies that need expensive temperature- and pressure-resistant equipment.
Obviously, the new processing methods open numerous prospects both for fundamental academic science and practical application.
Alexey Bilyachenko, Deputy Director, Research Institute of Chemistry, RUDN University