Nov 28 2017
Under the environmental concerns such as greenhouse effects and pollution, environment-friendly energy storage applications such as ammonia production, fuel cells and lithium-air batteries are proposed to substitute fossil resources.
However, the high overpotential is one of the most pressing issues for the practical applications, and electrocatalysts are used to lower overpotential. There are certain difficulties to design high-activity catalysts for electrochemical conversions. Researchers from State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, China and Materials Genome Institute, Shanghai University, Shanghai, China reviewed a few representative activity descriptors to screen high-activity catalysts in future high-throughput experiments and calculations. This research, titled "Adsorption-Energy-Based Activity Descriptors for Electrocatalysts in Energy Storage Applications", was published in National Science Review, 2017.
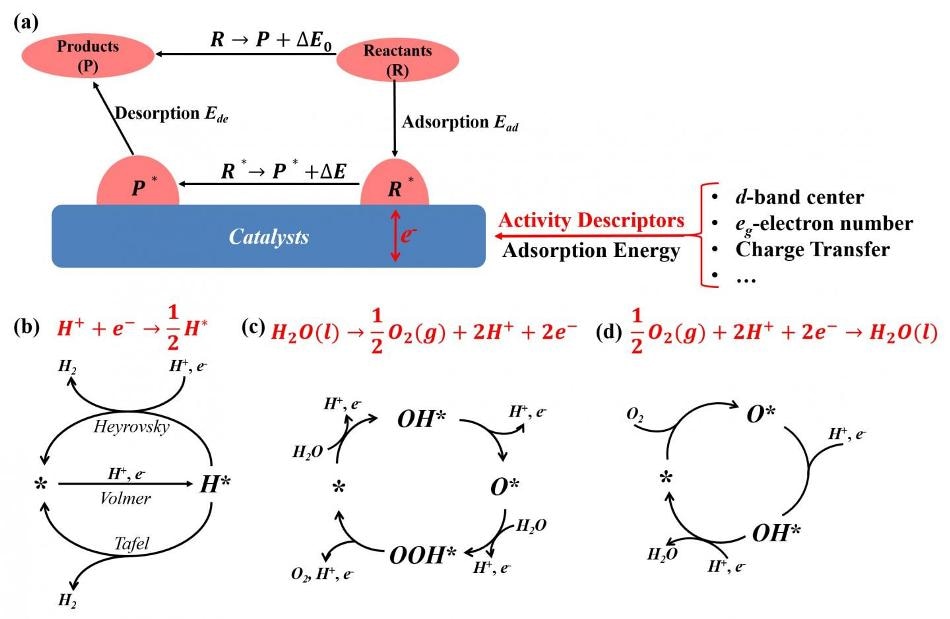 (a) Different activity descriptors for electrochemical reactions and catalytic cycles of (b) hydrogen evolution reaction, (c) oxygen evolution reaction and (d) oxygen reduction reaction in fuel cells. (CREDIT - ©Science China Press)
(a) Different activity descriptors for electrochemical reactions and catalytic cycles of (b) hydrogen evolution reaction, (c) oxygen evolution reaction and (d) oxygen reduction reaction in fuel cells. (CREDIT - ©Science China Press)
These researchers outline a simple approach to enhance catalytic activity to decrease activation barriers of electrochemical reactions by tweaking interfacial electronic coupling between the adsorbate and catalyst surface.
"The electrocatalytic processes usually involve the adsorption of reactants on the surfaces of catalysts, break some reactant bonds to form new chemical bonds between the catalyst and reactants, and result in activated intermediates. Because the catalytic activity is attributed to the interfacial electronic coupling, adsorption energy is a good descriptor to identify catalytic activity for surface reactions."
Based on the free energy change of electrochemical reaction, the authors separated the entire electrochemical reaction into a catalytic effect part and an intrinsic reaction part.
The catalytic effect is directly reflected in the adsorption energy differences of reactants and products.
The Authors
Adsorption energy as a catalytic descriptor in those usual reactions is comprehensively discussed in on-electron pair reactions, reduction reactions, and evolution reactions to present the effect of electronic coupling between charged species and catalysts on catalytic activity.
"The relationship between adsorption energy and catalytic activity is helpful for the initial selection of catalysts and the key of mapping the relationship is to establish the quantitative relationship between the intrinsic electronic properties of materials and catalytic descriptors." they emphasized.
Structural and elementary descriptors such as tolerance factor, d-band center and eg electron number are described in d-band framework related to adsorption energy. "Furthermore, because structural and elementary descriptors are experimentally quantified compared with adsorption energy, structural and elementary descriptors are useful to discover new catalyst materials and ensure a leap forward in electrochemical performance."
Charge transfer is also an important part in electrochemical reactions and improve the catalytic activity. The principle of charge transfer is to remove charge from stable bonds in reactants and lower the activation barrier of the rate-limiting step.
The Authors
"Fundamental understanding of structure-activity relationships between catalytic activity and physical properties of catalyst materials is helpful to choose effective descriptors and to develop efficient multiscale computational models for an accurate description of catalyst materials. High-throughput calculations and experiments can be employed to speed up the screening of catalyst materials and shorten the development cycle in the future studies", the scientists forecast. "However, even solving the intrinsic problem of activity, catalysts still face the vital requirements of stability and safety before practical applications", they underline, "Those stability and safety issues also should be considered before one catalyst, which is screened to perform high catalytic activity, is put into application."