Apr 25 2018
The fundamental mechanisms dictating the conversion of sunlight into energy in hybrid perovskite materials have been revealed in real time by neutron scattering. A deeper knowledge of this behavior will allow manufacturers to develop solar cells that have superior efficiency.
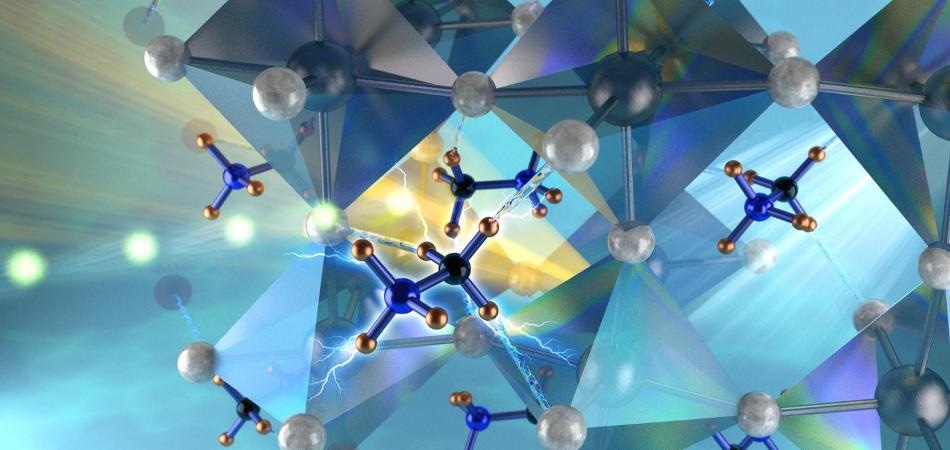 Neutron interactions revealed the orthorhombic structure of the hybrid perovskite stabilized by the strong hydrogen bonds between the nitrogen substituent of the methylammonium cations and the bromides on the corner-linked PbBr6 octahedra. (Image credit: ORNL/Jill Hemman)
Neutron interactions revealed the orthorhombic structure of the hybrid perovskite stabilized by the strong hydrogen bonds between the nitrogen substituent of the methylammonium cations and the bromides on the corner-linked PbBr6 octahedra. (Image credit: ORNL/Jill Hemman)
The multi-institutional research team from the Department of Energy’s Oak Ridge National Laboratory, Hunan University, and the University of Nebraska–Lincoln adopted photoluminescence measurements, together with neutron and x-ray scattering, to assess the link between the optoelectronic properties and microscopic structure of the material. The researchers could track atomic structural changes and determine the significance of hydrogen bonding in the performance of the material by studying the material under varying degrees of temperature. Their findings have been reported in the Advanced Materials journal.
Hybrid perovskites may prove to be more efficient in the conversion of light into energy when compared to the conventional solar cell materials. Moreover, they are easier to produce since it is possible to spin cast them from solution without requiring high-vacuum chambers for synthesis.
Hybrid perovskites, in contrast to their singular germanium or silicon equivalents, consist of inorganic molecules as well as organic molecules. The structure is formed from the arrangement of bromine and inorganic lead molecules in octahedral units, forming cages around the organic methylammonium cations (positively charged ions) made up of hydrogen, nitrogen, and carbon.
The advantage of having both organic and inorganic molecules in a well-defined crystal structure means we can tailor the material by tuning either one group or the other to optimize the properties. But even though researchers have been studying these materials for several years, we still don’t fully understand on a fundamental level how the organic components are affecting the properties.
Kai Xiao, Researcher - ORNL’s Center for Nanophase Materials Sciences
In order to unlock more functionality, finding the suitable molecular orientation and combination of the organic/inorganic components is essential. However, the right tools are necessary to understand those interactions.
Neutrons are very good at this because they’re sensitive to lighter elements like hydrogen. Because we’re able to track each neutron, we get information about things like where the atoms are, what their temperature is, and how they’re behaving.
Xiaoping Wang, ORNL Instrument Scientist
It was possible for the team to observe the hydrogen bonding interactions at the atomic scale using the TOPAZ instrument at ORNL’s Spallation Neutron Source.
The experiments showed that the material sustains considerable structural changes between roughly 150 and 130 K (approximately −190 and −225 °F). The movement of the organic component slowed down into an ordered state when the material was cooled. In the ordered state, precise in situ measurements were performed in real time to accurately study the process by which organic molecules were binding to the lead-bromine component through hydrogen bonds.
We saw the ordering is directly related to the hydrogen bonding in the structure, and how any changes can affect the energy gap of the material. That lets us know how well sunlight is being absorbed and what that could mean in terms of applications for photovoltaic materials.
Xiaoping Wang, ORNL Instrument Scientist
Complementary x-ray scattering and photoluminescence measurements, combined with crystal synthesis, were performed at CNMS. Researchers at ORNL’s Materials Science and Technology Division carried out theoretical calculations.
“Hybrid perovskites are already a good material,” stated Xiao. “Now that we know how the orientation of the organic molecules impacts the crystal structure, and how we can tune them further to change the desired properties, this new fundamental understanding will enable us to design new materials with even greater potential.”
Among Xiao and Wang’s coauthors were lead author Bin Yang, Wenmei Ming, Mao-Hua Du, Jong K. Keum, Alexander A. Puretzky, Christopher M. Rouleau, Jonsong Huang, and David B. Geohegan.
DOE’s Office of Science supported the study. The Spallation Neutron Source and the Center for Nanophase Materials Sciences are DOE Office of Science User Facilities. ORNL is managed by UT-Battelle for the Department of Energy’s Office of Science, which is the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science endeavors to address some of the most vital challenges faced by this generation of researchers.