Jun 25 2018
Chinese scientists have created new magnetic Janus particles to facilitate oil-water separation. These particles, known as hydrophilic/oleophilic magnetic Janus particles, can separate micro-scaled oil droplets from water quickly and efficiently.
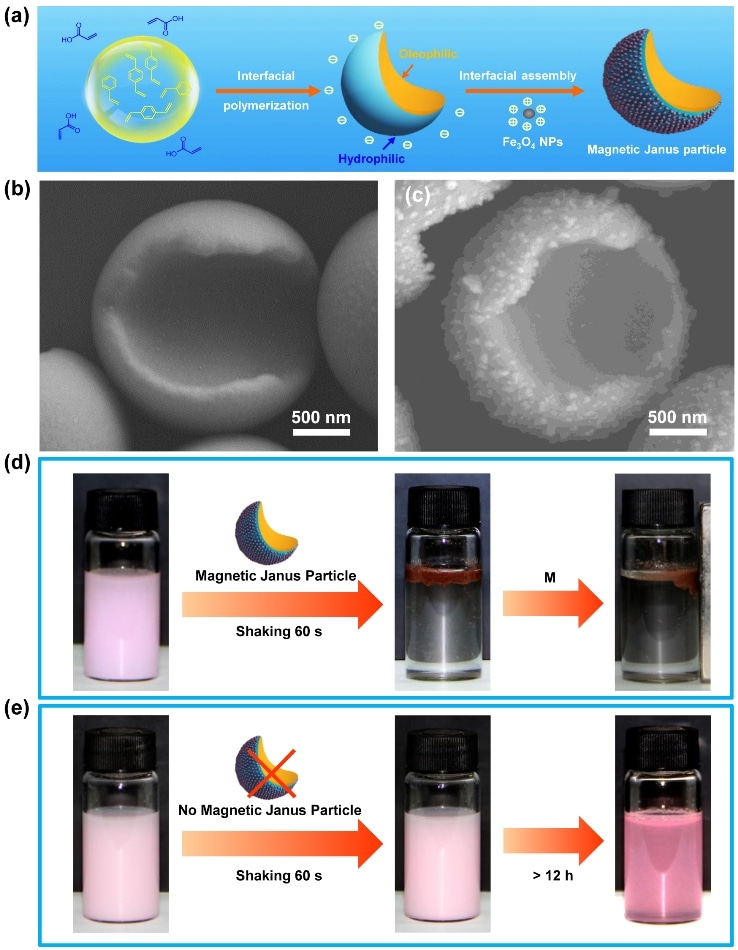 Hydrophilic/oleophilic magnetic Janus particles. (a-c) Synthesis. (d-e) Rapid and efficient oil-water separation. Image Credit: Dr. SONG Yongyang.
Hydrophilic/oleophilic magnetic Janus particles. (a-c) Synthesis. (d-e) Rapid and efficient oil-water separation. Image Credit: Dr. SONG Yongyang.
Oil pollution has become a universal challenge. In reality, oils are typically classified as surfactant-stabilized oil droplets, floating oils, dispersed oils, and surfactant-free micro-scaled minute oil droplets.
In the past few years, a number of efforts via control of the surface wettability towards realizing the effective separation of dispersed oils, floating oils, and even surfactant-stabilized oil droplets have been undertaken. However, the separation of micro-sized oil droplets from water has frequently been ignored.
Micro-scaled oil droplets measuring less than 20 μm in diameter are highly stable in water. A number of challenges remain when using traditional approaches and superwettable materials to separate these micro-scaled oil droplets from water.
Now, scientists from the Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (TIPCCAS) have formulated an emulsion interfacial polymerization method to synthesize anisotropic Janus particles possessing controllable topological and chemical anisotropy.
This technique can also be expanded to large-area fabrication of 2D Janus film actuators.
On the foundation of these studies, the team recently synthesized hydrophilic/oleophilic magnetic Janus particles using emulsion interfacial polymerization and the interfacial assembly of Fe3O4 nanoparticles, realizing the fast and efficient separation of micro-scaled minute oil droplets from water.
When incorporating the hydrophilic/oleophilic magnetic Janus particles into the oil-in-water emulsion and then shaking for one minute, the originally stable emulsion quickly formed layers.
After regulating with a magnetic field, the upper oil phase could be quickly attracted toward the magnet. The entire process was done within two minutes. The separation realized a high efficiency (>99%) and is relevant to a variety of oils and numerous oil contents.
Scientists also discovered that the oleophilic surface of Janus particles was advantageous for trapping a large number of minute oil droplets to make them coalesce. Lastly, like surfactant molecules, these Janus particles could adsorb at the interface of large oil droplets to make them stable.
It is possible to self-assemble these Janus particles on the interface of the larger oil droplets with their hydrophilic convex surface toward the water phase and their oleophilic concave surface toward the oil phase.
These particles offer a worthy candidate for application in the clean-up of industrial wastewater and water purification.