Sep 3 2018
Strange trans-selectivity is generated in hydroboration of alkynes when a combination of organoboron and radical chemistry is used. For this chemical transformation to be successful, it is vital to use N-heterocyclic carbene boranes. It is anticipated that this research will pave the way for the development of innovative boron-containing materials.
 This is an overview of the present research. (Image credit: Kanazawa University)
This is an overview of the present research. (Image credit: Kanazawa University)
Background
Organoboron compounds have been extensively used for the development of innovative organic molecules since Professor Suzuki, a 2010 Nobel Prize winner in chemistry, formulated palladium-catalyzed cross coupling reactions with organoboron compounds (Suzuki coupling). Moreover, various boron-containing compounds are promising materials in their own right. They can be used as organic electro-luminescence materials and medicines due to boron’s distinctive electronic nature.
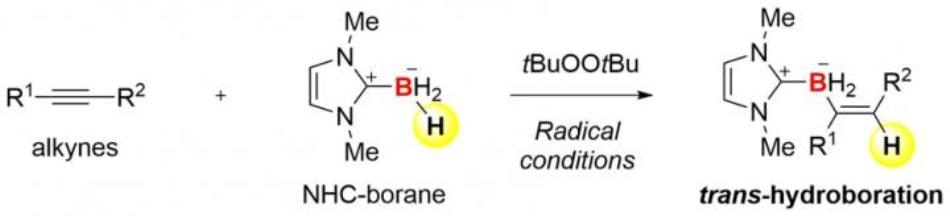
Borane (BH3) and its derivatives that are stabilized by ligands are the simplest boron compounds. They have the ability to react with a carbon‒carbon triple bond (alkyne, C≡C) to form an insertion product (H‒C=C‒BH2). Although this reaction, known as hydroboration, is a robust technique for the synthesis of organoboron compounds, it typically produces only a cis-product, which suggests that H and BH2 are introduced on the same side of the triple bond. In other words, it is hard to produce a trans-hydroboration product with the opposite geometric configuration. Earlier, there have been very few examples of trans-hydroboration of alkynes.
Results
The collaboration between Kanazawa University and the University of Pittsburgh proved successful in formulating trans-hydroboration reactions of alkynes based on radical chemistry. The researchers used N-heterocyclic carbene boranes (NHC-boranes) to merge hydroboration with radical chemistry. It is easy to handle NHC-boranes due to their stability. They are also better precursors of boryl radicals (boron-centered radicals). In fact, it is possible to readily form an NHC-boryl radical through simple thermolysis in the presence of low-cost commercial di-tert-butyl peroxide.
A carbon‒boron (C‒B) bond and a new carbon radical can be formed by adding the radical to an alkyne. Kinetic inducing of trans-selectivity in hydroboration is achieved when a hydrogen atom from the starting NHC-borane is captured by the highly reactive carbon radical. Consequently, a radical chain cycle is established by the overall process. This mechanism is very different from that of familiar hydroboration reactions.
From easily available alkynes, the current protocol offers a number of bench-stable alkenyl borane compounds that cannot be easily accessed through known techniques. A few of them can be transformed into retinoid mimics, which are drug candidates, by modified Suzuki coupling.
Significance and Future Prospects
Organoboron compounds produced using the existing technique will provide access to innovative boron-containing π-systems by further chemical transformation. Hence, this type of trans-hydroboration reaction will enable developments in medicinal chemistry and materials science. From the point of view of pure chemistry, this research increases the ability of radicals in synthetic chemistry. In summary, this study shows that radicals have the ability to control chemical reactions in a precise manner in spite of their extreme reactivity.
It has been a pleasure to be involved in this collaboration, which was led by the Kanazawa team. The new reaction that we have discovered is unique, and I am excited about its prospects for extension in the directions of both basic research and practical applications.
Dennis Curran (University of Pittsburgh), Collaborator