Dec 6 2018
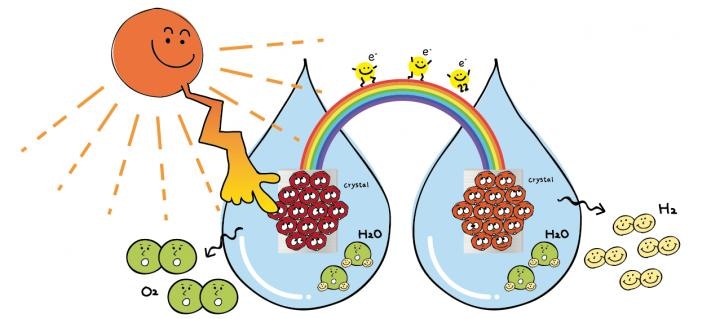
A study by a collaborative group of researchers from Japan could help humans reach a step closer to hydrogen-powered electronics, travel, and much more. The group has devised a new technique for more efficient production of a component key to the conversion of water and solar energy into hydrogen fuel, a process known as photoelectrochemical water splitting.
 Photoelectrochemical water splitting using “flux-grown photo anode” to convert solar energy and water into hydrogen fuel efficiently. (Image credit: Katsuya Teshima PhD, the director of the Center for Energy and Environmental Science, Shinshu University)
Photoelectrochemical water splitting using “flux-grown photo anode” to convert solar energy and water into hydrogen fuel efficiently. (Image credit: Katsuya Teshima PhD, the director of the Center for Energy and Environmental Science, Shinshu University)
The outcomes of the study were reported in Applied Energy Materials, a journal of the American Chemical Society, in October 2018.
With the abundance of solar energy and water, photoelectrochemical water splitting is a promising way to ease global environment and energy-storage issues.
Katsuya Teshima, Study Lead Author, Shinshu University.
Teshima is a professor of the Department of Materials Chemistry and the director of the Center for Energy and Environmental Science at Shinshu University. He is also affiliated with the Nagano Prefecture Nanshin Institute of Technology.
Water splitting involves the use of a photo anode, which is a metal cathode and a semiconductor, to absorb sunlight. From that light, the semiconductor absorbs high-energy photons, thereby inducing the splitting of the molecules around the semiconductor. This results in oxygen leaving hydrogen and combining with other free oxygen molecules. Then, oxygen and hydrogen pairs can be separately funneled through to the suitable cathodes to be stored and used as energy.
Teshima and Suzuki, a coworker, state that the challenge, however, is that the photo anodes first proposed were able to absorb only UV light, which accounts for about 5% of the solar spectrum. These photo anodes are made of titanium oxide and are highly efficient at transforming whatever solar energy they absorb. However, they are not a practical option for industrial use since they absorb very little solar energy.
Teshima and his group have opted to use tantalum nitride, one of the most favorable light-responsive materials available for use in water splitting. Apart from absorbing visible light, it can also absorb light with a wavelength of up to 600 nm, thereby enabling even more light absorption. Although the scientists fabricated the tantalum nitride crystals earlier, the process was quite complex and the ensuing crystal layer varied in coverage and thickness. Such asymmetry can result in inefficient or even totally ineffective water splitting attempts.
As part of the fresh effort, Teshima positioned the metal tantalum samples on top of powder sodium compounds and used ammonia gas to heat them at high temperatures. The team was able to control the extent to which the sodium compounds evenly reacted with the tantalum, and even the thickness to which the crystal layer grew, by varying the temperature, the ratio of the sodium compounds, and the time.
Our ultimate goal is to efficiently produce hydrogen and oxygen gasses from natural water by use of our flux-grown photo anode. As environment and energy problems are global issues, we want to contribute to their solutions.
Katsuya Teshima, Study Lead Author, Shinshu University.
This study was supported by Grants-in-Aid for Scientific Research, Grants-in-Aid for Young Scientists, and the Japan Technological Research Association of Artificial Photosynthetic Chemical Process.