Jan 22 2019
The Departments of Chemistry at the University of Oxford (United Kingdom) and University of Jyväskylä (Finland) have made a collaborative research effort that has led to the discovery of a gold compound that demonstrates nucleophilic behavior, which has been unknown for molecular gold until now.
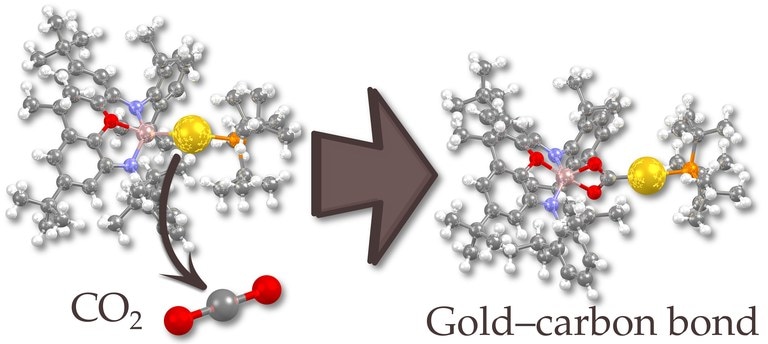 A novel aluminum-gold compound (left) reacts with carbon dioxide by insertion (right) showcasing the nucleophilic character of the gold atom. (Image credit: University of Jyväskylä)
A novel aluminum-gold compound (left) reacts with carbon dioxide by insertion (right) showcasing the nucleophilic character of the gold atom. (Image credit: University of Jyväskylä)
The study opens the door for new opportunities in using gold compounds, for instance, as catalysts in innovative chemical reactions.
A wide range of transition metal compounds includes a positively charged metal ion that is attached to negatively charged or neutral electron donor compounds. The metals in these complexes function as electron acceptors (electrophiles). Gold is unique in this respect since it is the only transition metal to produce a negatively charged “naked” ion (auride) in solid or solution state. Hence, principally, gold can also play the role of an electron donor (nucleophile). Yet, the first example of nucleophilic reactivity exhibited by molecular gold system in solution has been reported only now.
New Reaction to Gold
The linear two-coordinate compound thus produced exhibits an aluminum-gold bond that is increasingly polarized toward gold as anticipated based on the considerably greater potential of the gold atom to attract electrons.
This transfer of electron-rich character from aluminium to gold is highlighted by the reactivity of the complex: a reaction between the compound and carbon dioxide results in a product where the gold atom has attacked the central carbon atom.
Petra Vasko, Postdoctoral Researcher, University of Jyväskylä
It is a well-known fact that gold complexes act as powerful electrophiles in various useful catalytic transformations. Moreover, only now has text-book transition metal reactivity (migratory insertion, oxidative addition, etc.) been started to be developed for gold.
Nucleophilic behaviour has been virtually non-existent prior to this work. Hence, these results can open up a completely new facet in gold chemistry.
Akseli Mansikkamäki, Postdoctoral Researcher, University of Jyväskylä
The study has been published in Springer Nature Chemistry on January 21st, 2019.