Mar 1 2019
Silicate glass is used in numerous applications, including as a nuclear waste form to restrain radioactive elements from spent fuel. However, it has one drawback—it corrodes when it is exposed to aqueous solutions. Researchers at the University of Bonn were able to study in detail which processes occur. The findings can be found in the journal Nature Materials.
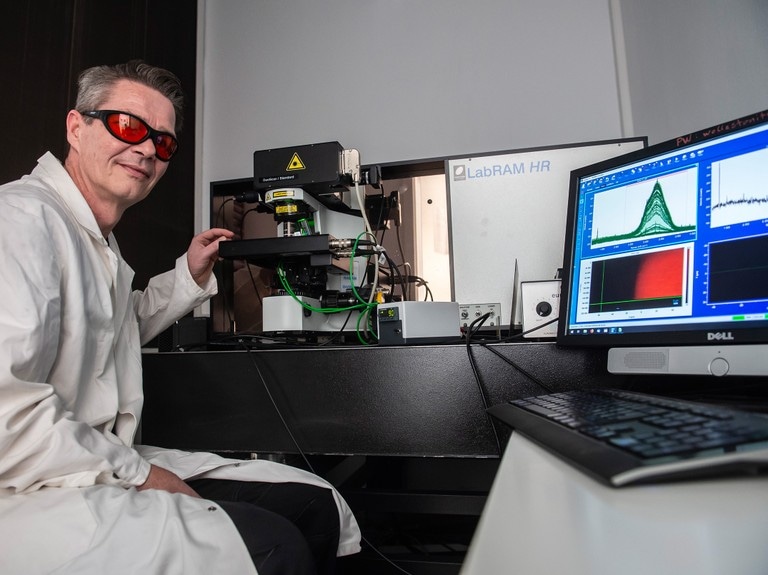 Detailed insight into the corrosion of glass: Prof. Dr. Thorsten Geisler-Wierwille from the Institute for Geosciences and Meteorology at the Raman spectrometer with built-in heating vessel. (© Photo: Barbara Frommann/Uni Bonn)
Detailed insight into the corrosion of glass: Prof. Dr. Thorsten Geisler-Wierwille from the Institute for Geosciences and Meteorology at the Raman spectrometer with built-in heating vessel. (© Photo: Barbara Frommann/Uni Bonn)
The so-called confocal Raman spectroscopy was used by the mineralogists and geochemists at the University of Bonn for their study. Here, a laser beam targets a sample through a microscope. The light works together with the molecules in the material, making them vibrate. Individually backscattered photons alter their color based on the sample’s structure and the chemical properties. This occurrence is known as the Raman Effect. The initially monochromatic light currently also has other color components. The color spectrum offers thorough insights into the composition and structure of the matter that is stimulated by the laser beam.
What makes this technique even more remarkable: The laser can be targeted to a particular point in the space with an accuracy of a few thousandths of a millimeter. This enables exploring the sample point by point, but not only on its surface: If the sample is transparent, the beam can also be aimed at internal areas. “And that's exactly what we did,” explains Prof. Dr. Thorsten Geisler-Wierwille from the Institute for Geosciences and Meteorology at the University of Bonn.
Opal layer at the glass surface
A small piece of silicate glass was used as a sample by the scientists. The sample reacted with an aqueous solution in a specifically developed heating vessel. The vessel could be moved in steps of one-thousandth of a millimeter under the Raman microscope—to the left, right, forward, and backward, but also up and down. “We scanned the glass point by point and recorded a Raman spectrum while it reacted with the solution,” says Lars Dohmen, who is presently finishing his doctorate under the supervision of Geisler-Wierwille. “This allowed us to investigate the reaction almost in real time. This currently works at temperatures of up to 150 degrees, which, for instance, are also expected in a nuclear repository.”
The results show that silicate glass rapidly dissolves when it touches aqueous solutions—virtually like a sugar cube in a cup of coffee. However, while the sugar molecules are rapidly dispersed evenly in the water by diffusion, this is not the case with glass corrosion: Part of the resulting dissolved silica appears to stay near the surface of the glass. At a specific point, its concentration becomes so high that it hardens.
“We then also speak of silica precipitation,” explains Prof. Geisler-Wierwille. “Silica molecules in the solution interlink to form aggregates only a few millionths of a millimeter in size, which are deposited at the glass surface and mature into an opal-like state.” However, the team was able to demonstrate that this opal layer does not provide ideal protection against water. Rather, the dissolution-precipitation front continues to corrode its way into the glass. Consequently, the glass is slowly replaced by opal, although at a declining velocity.
For the first time, we have experimentally demonstrated that a boundary solution with dissolved silica forms between the opal layer and the underlying glass. As the thickness of the opal layer increases, it increasingly prevents the silica solution from being transported away from the reaction interface. We suspect that it eventually gels to a viscous mass, which dramatically slows down glass dissolution.
Dr. Thorsten Geisler-Wierwille, Professor, Institute for Geosciences and Meteorology, University of Bonn.
In the research, this was already the circumstance after 25 thousandths of a millimeter. “Even though the reaction became very slow, it cannot be ruled out that this corrosion process will release radioactive elements over long periods of time,” stresses Geisler-Wierwille. However, glasses used for the vitrification of nuclear waste are undoubtedly steadier against water than the examined glass. “We want to extend our experiments to these glass types in the near future,” stresses the researcher. Studies with silicate glass wherein radioactive elements are already added are also in the pipeline. The scientists and their partners want to examine the impact of self-irradiation damage in the glass on its resistance to corrosion.
The current work should mainly prove that our new method can provide far-reaching insights into these processes.
Dr. Thorsten Geisler-Wierwille, Professor, Institute for Geosciences and Meteorology, University of Bonn.
The level of interest exhibited by industry in this study is also reflected in the sponsoring of the pilot project: One of the sponsors of the research is the well-known glass manufacturer Schott AG.