Apr 23 2019
As people become progressively dependent on batteries for everything from electric vehicles to portable devices, the major challenge to enhance energy storage and boost battery life, while guaranteeing safe and reliable operation, is turning out to be increasingly important.
 Schematics of the protection mechanism of boron nitride (BN) and characterizations of BN nanofilm. The left visual shows that a Lithium aluminum titanium phosphate (LATP) pellet that touches lithium metal will be immediately reduced. The severe side reaction between lithium and solid electrolyte will fail the battery in several cycles. The right shows that an artificial BN film is chemically and mechanically robust against lithium. It electronically isolates LATP from lithium, but still provides stable ionic pathways when infiltrated by polyethylene oxide (PEO), and thus enables stable cycling. (Image credit: Qian Cheng/Columbia Engineering)
Schematics of the protection mechanism of boron nitride (BN) and characterizations of BN nanofilm. The left visual shows that a Lithium aluminum titanium phosphate (LATP) pellet that touches lithium metal will be immediately reduced. The severe side reaction between lithium and solid electrolyte will fail the battery in several cycles. The right shows that an artificial BN film is chemically and mechanically robust against lithium. It electronically isolates LATP from lithium, but still provides stable ionic pathways when infiltrated by polyethylene oxide (PEO), and thus enables stable cycling. (Image credit: Qian Cheng/Columbia Engineering)
Headed by Yuan Yang, assistant professor of materials science and engineering, researchers from Columbia Engineering have recently reported that they have come up with a new technique to safely extend the life of batteries. In this technique, a nano-coating of boron nitride (BN) is inserted to stabilize solid electrolytes present in lithium metal batteries. The results have been described in a recent study published by Joule.
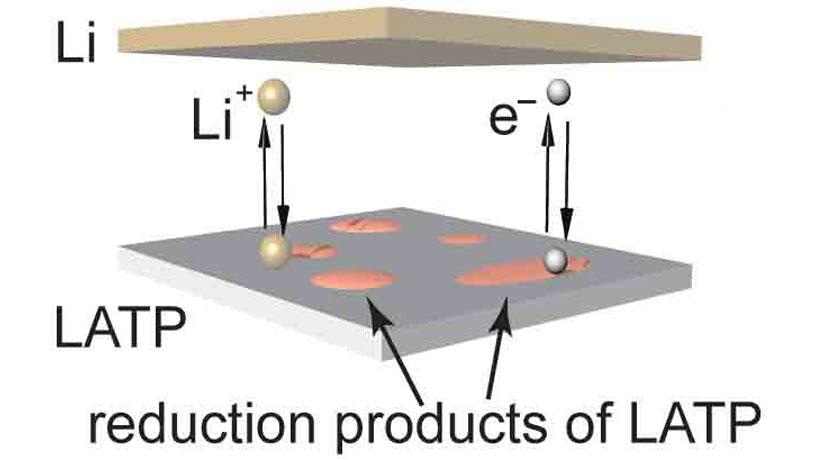
Currently, traditional lithium-ion (Li-ion) batteries are extensively utilized in everyday life; however, these batteries possess low energy density, leading to relatively reduced battery life. Moreover, the presence of highly flammable liquid electrolyte within these batteries can cause them to short out and also catch fire.
Better energy density can be realized by substituting the graphite anode utilized in Li-ion batteries with lithium metal: the theoretical capacity of the lithium metal for the amount of charge it can deliver is nearly 10 times greater when compared to graphite. However, dendrites are usually formed at the time of lithium plating, and when these dendrites enter the membrane separator in the center of the battery, they can generate short-circuits, thus raising concerns regarding battery safety.
We decided to focus on solid, ceramic electrolytes. They show great promise in improving both safety and energy density, as compared with conventional, flammable electrolytes in Li-ion batteries. We are particularly interested in rechargeable solid-state lithium batteries because they are promising candidates for next-generation energy storage.
Yuan Yang, Assistant Professor, Materials Science and Engineering, Department of Applied Physics and Applied Mathematics, Columbia University
A majority of solid electrolytes are ceramic, and hence they are non-flammable, removing safety concerns. Solid ceramic electrolytes also possess a high mechanical strength that can, in fact, suppress the growth of lithium dendrites, rendering lithium metal a coating choice for battery anodes. Yet, a majority of solid electrolytes are not stable against Li, because lithium metal corrodes them easily, making them unsuitable for use in batteries.
Lithium metal is indispensable for enhancing energy density and so it’s critical that we be able to use it as the anode for solid electrolytes. To adapt these unstable solid electrolytes for real-life applications, we needed to develop a chemically and mechanically stable interface to protect these solid electrolytes against the lithium anode. It is essential that the interface not only be highly electronically insulating, but also ionically conducting in order to transport lithium ions. Plus, this interface has to be super-thin to avoid lowering the energy density of batteries.
Qian Cheng, Study Lead Author and Postdoctoral Research Scientist, Department of Applied Physics and Applied Mathematics, Columbia University
Cheng works in Yang’s group.
In order to address these challenges, the researchers worked with coworkers at the City University of New York and Brookhaven National Lab. They performed an experiment in which 5~10 nm boron nitride (BN) nano-film was deposited as a protective layer in order to separate the electrical contact between the ionic conductor (the solid electrolyte) and the lithium metal, together with a trace amount of liquid electrolyte or polymer to infiltrate the electrolyte/ electrode interface.
Eventually, BN was chosen as a protective layer because it is mechanically and chemically stable with lithium metal, offering excellent electronic insulation. The team fabricated the BN layer to possess intrinsic defects, via which lithium ions can easily travel through, enabling it to act as an outstanding separator. Moreover, a chemical vapor deposition technique can be used to readily prepare the BN layer to create atomically thin scale (~nm level), large-scale (~dm level), and continuous films.
While earlier studies used polymeric protection layers as thick as 200 µm, our BN protective film, at only 5~10 nm thick, is record-thin—at the limit of such protection layers—without lowering the energy density of batteries. It’s the perfect material to function as a barrier that prevents the invasion of lithium metal to solid electrolyte. Like a bullet-proof vest, we’ve developed a lithium-metal-proof ‘vest’ for unstable solid electrolytes and, with that innovation, achieved long-cycling lifetime lithium metal batteries.
Qian Cheng, Study Lead Author and Postdoctoral Research Scientist, Department of Applied Physics and Applied Mathematics, Columbia University
Currently, the investigators are extending their technique to an array of unstable solid electrolytes and additionally improving the interface. They are hoping to develop solid-state batteries with long-cycle lifetimes and high performance.